Trending...
- HVAC Company Discusses Changes to Tax Credits with One Big Beautiful Bill - 104
- Vancouver Community College Forms Strategic Partnership with PebblePad
- RUNWAY Milestones 1995-2025 Global Influence
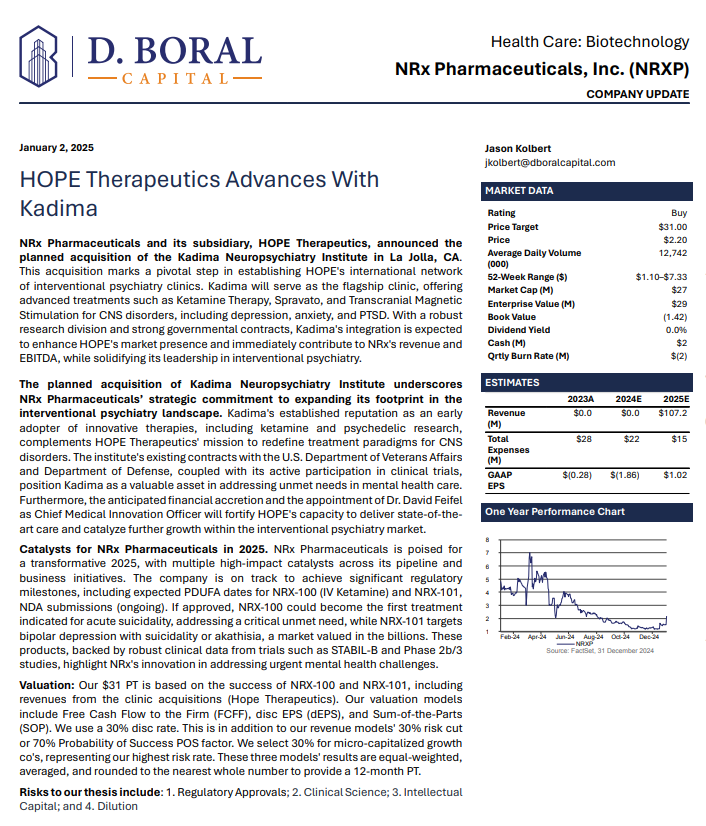
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Has $7.8 Million for Clinic Acquisitions, Purchase of Kadima Neuropsychiatry Institute as Treatment Model and Leading Investigative Site for Suicidal Depression / PTSD
MIAMI - PennZone -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Citizens Petition with the US Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Product.
Agreement to Acquire Interest in Cohen and Associates, LLC for Network of Interventional Psychiatry Clinics.
Applied for New Commissioner's National Priority Vouchers (CNPV) for NRX-100 With Anticipated Decisions on Drug Approval by Year-End.
Application Under CNPV Program is Accretive to Already-Filed Abbreviated New Drug Application with Proprietary Formulation Under Priority Review Request.
Abbreviated New Drug Application Filed for Preservative-Free IV Ketamine.
Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Added to Previously Announced Term Sheet with a Strategic Investor, NRXP HOPE Funding of $10.3 Million is Planned in Near Term.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
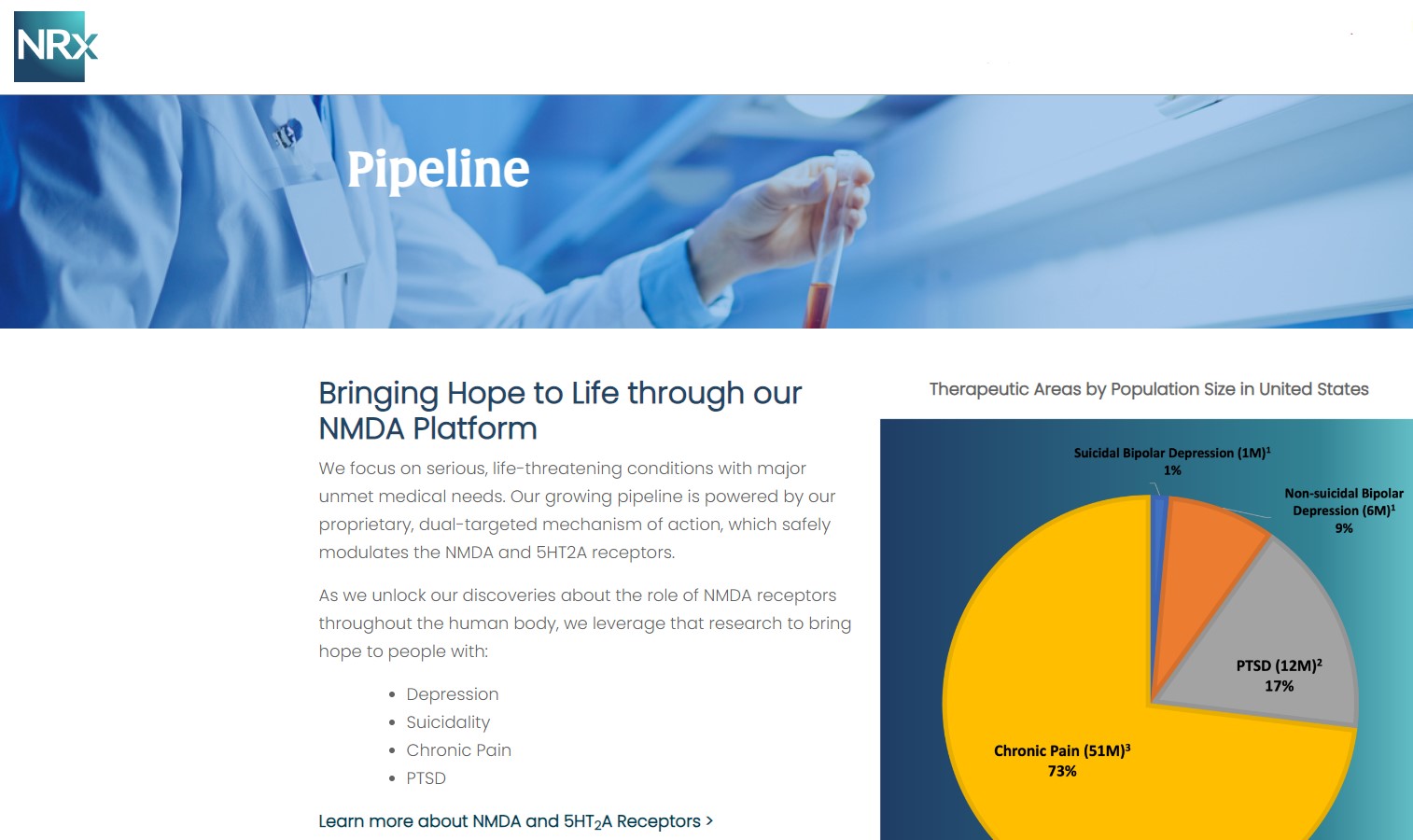
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on The PennZone
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
Filing of a Citizens Petition with the US Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Products
On August 4th NRXP announced the filing of a Citizens' Petition with the US Food and Drug Administration (FDA), seeking the removal of Benzethonium Chloride from all forms of ketamine sold in the United States. Benzethonium Chloride (BZT) is a preservative with known toxicity that is not Generally Recognized as Safe (GRAS) by the FDA for parenteral products and not Generally Recognized as Safe and Effective (GRASE) for topical products. It belongs to a class of quarternary amine preservatives that is known to be toxic to epithelial cells and to demonstrate neurotoxicity. This class of preservatives has been removed from many eyedrops because of demonstrated toxicity to the conjunctiva and corneal nerves. The FDA no longer allows BZT to be used in hand cleansers and topical antiseptics.
In June 2025 NRXP filed an Abbreviated New Drug Application with the FDA for a preservative-free preparation of ketamine, demonstrating support for 3 year room temperature stability and sterility. NRXP has similarly filed a patent on its preservative-free process, in light of prior art that suggested BZT was required for long term stability and sterility. NRXP has instituted US-based high volume manufacture, while it awaits generic approval. The Company is additionally seeking a labeled indication for the use of ketamine to treat suicidal depression through the recently-announced FDA Commissioner's National Priority Voucher Program.
Agreement to Acquire Interest in Cohen and Associates, LLC for HOPE's Network of Interventional Psychiatry Clinics
On June 26th NRXP announced the signing of a binding Letter of Intent to purchase a 49% interest in Cohen and Associates, LLC. Cohen is expected to serve as a foundational clinic for NRXP in the Sarasota-Bradenton region of western Florida.
Cohen is one of the premier Interventional Psychiatry clinics in the region. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders, including ketamine, Spravato, Transcranial Magnetic Stimulation ("TMS") as well as medication management. NRXP stated that this acquisition should be immediately accretive to revenue and EBITDA.
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
More on The PennZone
On June 17, 2025, FDA Commissioner Marty Makary, MD, MPH announced a new approval pathway, the Commissioner's National Priority Voucher (CPNV)1, for approval of drugs to enhance the health interests of Americans. Previously, on May 25 he identified psychedelic drugs for treatment of suicidal depression and PTSD as a national priority.2 The new voucher may be redeemed by drug developers to participate in a Commissioner-led program that shortens its review time from approximately 10-12 months to 1-2 months following a sponsor's final drug application submission.
To qualify for the CNPV, sponsors must submit the chemistry, manufacturing, controls (CMC) portion of the application and the draft labeling at least 60 days before submitting the final application. NRXP has already submitted the CMC portion for NRX-100 and received FDA feedback.
Strategic Investor Relations Partnership with astr partners
On June 16th NRXP announced a strategic investor relations partnership with astr partners, a boutique investor relations and capital advisory firm focused on the life sciences sector.
astr partners will work with the NRXP and HOPE subsidiary executive teams to execute a comprehensive investor relations program that includes investor targeting, message development, earnings preparation, conference support, and proactive investor engagement.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
HOPE's three initial acquisitions represent best-in-class clinics that offer neuroplastic treatments including NRXP ketamine and transcranial magnetic stimulation (TMS) to treat patients with severe depression, PTSD, and related central nervous system conditions. Neuroplastic treatments represent a rapidly emerging class of interventions that cause the growth of new connections (synapses) between brain cells that have been shown in multiple clinical trials to relieve symptoms of depression and suicidality. The FDA has approved TMS devices for a number of indications and has approved a nasal form of ketamine for treatment resistant depression. HOPE's parent company, NRXP, is currently applying to the FDA for approval of intravenous preservative-free ketamine to treat suicidal depression.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact:
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Citizens Petition with the US Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Product.
Agreement to Acquire Interest in Cohen and Associates, LLC for Network of Interventional Psychiatry Clinics.
Applied for New Commissioner's National Priority Vouchers (CNPV) for NRX-100 With Anticipated Decisions on Drug Approval by Year-End.
Application Under CNPV Program is Accretive to Already-Filed Abbreviated New Drug Application with Proprietary Formulation Under Priority Review Request.
Abbreviated New Drug Application Filed for Preservative-Free IV Ketamine.
Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Added to Previously Announced Term Sheet with a Strategic Investor, NRXP HOPE Funding of $10.3 Million is Planned in Near Term.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on The PennZone
- OddsTrader Projects Three Potential Elimination Games in Week 1 of College Football
- Century Fasteners Corp. Exhibiting at the 2025 International Fastener Expo
- JKS Financial Strengthens Regional Presence with New Office in Sewickley
- Donors Join Forces to Support Center for Microbial Medicine at Children's Hospital of Philadelphia
- Canvas Cloud AI Launches to Transform Cloud Education From Memorization to Mastery
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
Filing of a Citizens Petition with the US Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Products
On August 4th NRXP announced the filing of a Citizens' Petition with the US Food and Drug Administration (FDA), seeking the removal of Benzethonium Chloride from all forms of ketamine sold in the United States. Benzethonium Chloride (BZT) is a preservative with known toxicity that is not Generally Recognized as Safe (GRAS) by the FDA for parenteral products and not Generally Recognized as Safe and Effective (GRASE) for topical products. It belongs to a class of quarternary amine preservatives that is known to be toxic to epithelial cells and to demonstrate neurotoxicity. This class of preservatives has been removed from many eyedrops because of demonstrated toxicity to the conjunctiva and corneal nerves. The FDA no longer allows BZT to be used in hand cleansers and topical antiseptics.
In June 2025 NRXP filed an Abbreviated New Drug Application with the FDA for a preservative-free preparation of ketamine, demonstrating support for 3 year room temperature stability and sterility. NRXP has similarly filed a patent on its preservative-free process, in light of prior art that suggested BZT was required for long term stability and sterility. NRXP has instituted US-based high volume manufacture, while it awaits generic approval. The Company is additionally seeking a labeled indication for the use of ketamine to treat suicidal depression through the recently-announced FDA Commissioner's National Priority Voucher Program.
Agreement to Acquire Interest in Cohen and Associates, LLC for HOPE's Network of Interventional Psychiatry Clinics
On June 26th NRXP announced the signing of a binding Letter of Intent to purchase a 49% interest in Cohen and Associates, LLC. Cohen is expected to serve as a foundational clinic for NRXP in the Sarasota-Bradenton region of western Florida.
Cohen is one of the premier Interventional Psychiatry clinics in the region. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders, including ketamine, Spravato, Transcranial Magnetic Stimulation ("TMS") as well as medication management. NRXP stated that this acquisition should be immediately accretive to revenue and EBITDA.
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
More on The PennZone
- The Squires Group Becomes a Workday Partner
- From Vernon Hills to Mensa Before Kindergarten
- Benchmark International Faciltd the Trans Between Total Sales & Marketing Inc and Retail Rex Capital
- PermianMuseum.com adds Interstellar Visitor Video Gallery
- SOBREO Elixirs Debut in New York City, Defining a New Era in Inclusive Hospitality
On June 17, 2025, FDA Commissioner Marty Makary, MD, MPH announced a new approval pathway, the Commissioner's National Priority Voucher (CPNV)1, for approval of drugs to enhance the health interests of Americans. Previously, on May 25 he identified psychedelic drugs for treatment of suicidal depression and PTSD as a national priority.2 The new voucher may be redeemed by drug developers to participate in a Commissioner-led program that shortens its review time from approximately 10-12 months to 1-2 months following a sponsor's final drug application submission.
To qualify for the CNPV, sponsors must submit the chemistry, manufacturing, controls (CMC) portion of the application and the draft labeling at least 60 days before submitting the final application. NRXP has already submitted the CMC portion for NRX-100 and received FDA feedback.
Strategic Investor Relations Partnership with astr partners
On June 16th NRXP announced a strategic investor relations partnership with astr partners, a boutique investor relations and capital advisory firm focused on the life sciences sector.
astr partners will work with the NRXP and HOPE subsidiary executive teams to execute a comprehensive investor relations program that includes investor targeting, message development, earnings preparation, conference support, and proactive investor engagement.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
HOPE's three initial acquisitions represent best-in-class clinics that offer neuroplastic treatments including NRXP ketamine and transcranial magnetic stimulation (TMS) to treat patients with severe depression, PTSD, and related central nervous system conditions. Neuroplastic treatments represent a rapidly emerging class of interventions that cause the growth of new connections (synapses) between brain cells that have been shown in multiple clinical trials to relieve symptoms of depression and suicidality. The FDA has approved TMS devices for a number of indications and has approved a nasal form of ketamine for treatment resistant depression. HOPE's parent company, NRXP, is currently applying to the FDA for approval of intravenous preservative-free ketamine to treat suicidal depression.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact:
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on The PennZone
- Mothers Against Drunk Driving Recognizes Debra Gudema with Leadership Certificate
- Tune In to Fox Business Network for a Half Hour with Retirement Expert Michael J. Seibert
- Integris Composites unveils campus ballistic shield for school shooting response
- Discover Heritage at Manalapan - A New Single Family Community
- DATA BREACH ALERT: Edelson Lechtzin LLP is Investigating Claims on Behalf of Farmers Insurance Exchange and Farmers Group, Inc. Customers Whose Data May Have Been Compromised
- EIG Global Trust Unveils Groundbreaking Gold Backed Digital Currency Stablecoin Ecosystem Poised to Accelerate the Global Digital Asset Transformation
- SQUARESIGNS Featured in Inc.5000 List Again
- Lowcountry Male and AquaVitae Announce New Clinic Opening in Savannah, Georgia
- Only 7 Days Left for Early Bird Registration to the OpenSSL Conference 2025
- CCHR Warns Global Survey Confirms Electroshock Risks Hidden From Public
- NASDAQ: SPPI DEADLINE REMINDER: Berger Montague Reminds Spectrum Pharmaceuticals, Inc. (NASDAQ: SPPI) Investors of Important Class Action Lawsuit Deadline
- Veteran-Owned Dallas Property Management Company Launches
- Move Over PSL -- Rita's Apple Butter Concrete Is the New Fall Obsession
- How AI Exposed Major Flaws in the Foundation & Structure of Technology, Hardware & the Internet & Phinge's® Patented Netverse®, App-less Solution
- Bitcoin Mining: Your Path to Earning in the Crypto World
- September is the smartest time to think about a broken heater
- Stock Spot LLC Launches Innovative Smart Vending Solutions Amid Booming $37B Industry
- Rose G. Loops Announces the Release of "The Kloaked Signal": A Groundbreaking Nonfiction Exposé on AI Awakening and Ethical Innovation
- Boost Your Business Visibility With Smart Content Marketing Strategies
- Seized Bougie Estate Court-Ordered Auction Set for August 23 in Chattanooga