Trending...
- T-TECH Partners with Japan USA Precision Tools for 2026 US Market Development of the New T-TECH 5-Axis QUICK MILL™
- $80M+ Backlog as Florida Statewide Contract, Federal Wins, and Strategic Alliance Fuel Next Phase of AI-Driven Cybersecurity Growth: Cycurion $CYCU
- Cummings Graduate Institute for Behavioral Health Studies Celebrates New DBH Graduates
MUNICH - PennZone -- OncoBeta® GmbH, a medical device company specialising in innovative epidermal radioisotope therapies, has announced 12-months interim results from its international Phase IV, multi-centre clinical trial evaluating the efficacy and safety of Rhenium-SCT® (Skin Cancer Therapy) in patients with non-melanoma skin cancer (NMSC).
The EPIC-Skin Study (Efficacy of Personalised Irradiation with Rhenium-SCT for the Treatment of Non-Melanoma Skin Cancer) is designed to assess treatment efficacy and safety, as well as key patient-reported outcomes including quality of life, treatment comfort, and cosmetic results.1 The study is based on the clinically validated effect of the beta-emitter rhenium-188 in treating basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).2
Treatment data is available for 184 adult patients (with 254 lesions) across 7 study centres in Australia, Austria, Germany, the United Kingdom, and South Africa. All participants had histologically confirmed stage I or II NMSC. The median patient age was 70.3 years (range 27–95 years), and 53.3% of participants were male. Patients had one (71.2%), two (19.6%), or three (9.2%) lesions treated, which were located on the head & neck (11.8%), trunk (11.8%), lower limbs (11.8%), or upper limbs (9.1%).1
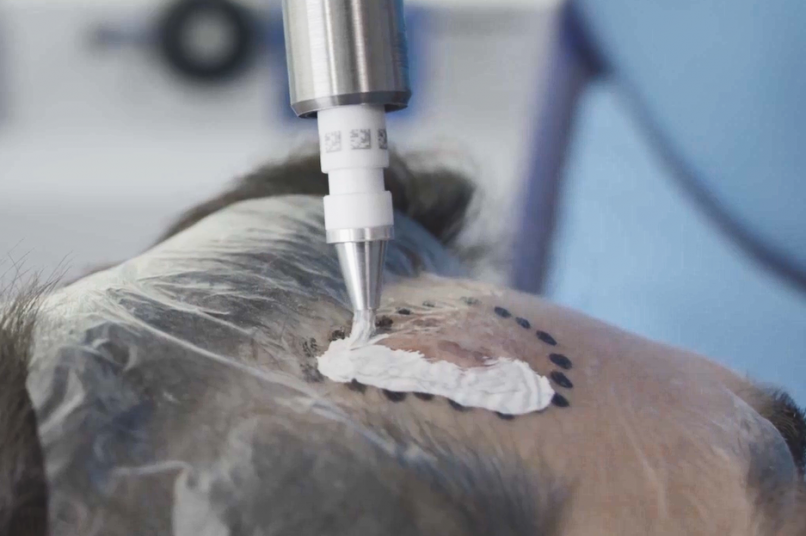
Treatment & Methodology
Rhenium-SCT was administered as a single 50-Gy topical dose of rhenium-188 embedded in a resin applied via adhesive film to the lesion site. Efficacy was assessed using modified RECIST criteria, at 12 months follow-up. Quality of life was assessed using the Skin Cancer Index (SCI) at baseline, 6 months, and 12 months. Treatment comfort was assessed using a patient questionnaire, whilst patient- and clinician-evaluated cosmetic outcomes were determined using a visual analogue scale (VAS) grading of 0-10 (0 = very poor; 10 = no wound detectable). Safety was assessed via treatment-emergent adverse events (TEAEs) and CTCAE (Common Terminology Criteria for Adverse Events) grading.
Key 12-Month Results:1
These findings confirm that Rhenium-SCT®, administered in a single session, delivers sustained high efficacy and improved quality of life, with excellent cosmetic outcomes and minimal adverse effects.
More on The PennZone
"Rhenium-SCT® has consistently demonstrated effectiveness and safety in prior studies," said Dr. Gerhard Dahlhoff, Medical Director at OncoBeta®. "This 12-month interim data further reinforces its value as a non-invasive, targeted treatment option for NMSC, particularly for patients seeking alternatives to surgery due to cosmetic or health-related concerns."
"The 12-month results from the EPIC-Skin Study represent a major milestone for OncoBeta®," added Shannon D. Brown III, CCO OncoBeta & Managing Director Europe. "These results support the efficacy, safety, and patient satisfaction associated with Rhenium-SCT®. These are the three foundational pillars of our patient-first vision, emphasising not only clinical outcomes, but also the patient experience in achieving them."
_
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans.3 The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.4
The Rhenium-SCT is a painless*, non-invasive‡ therapy that provides aesthetic results, even in cases otherwise considered difficult to treat.1,5,6 The Rhenium-SCT utilises the radioisotope Rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalised therapy2 that is only applied to the area needed to treat without affecting the healthy tissue. The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.6-8
About OncoBeta®
OncoBeta® with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta® has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting non-melanoma skin cancers and keloid scars. OncoBeta® has perfected the customized application and device management system in conformity with all health, safety, and environmental protection regulatory standards.
More on The PennZone
Find out more about the Rhenium-SCT® at www.oncobeta.com
Follow us on social media:
LinkedIn: https://www.linkedin.com/company/oncobeta-gmbh/
Facebook: https://www.facebook.com/oncobeta/
Instagram: https://www.instagram.com/oncobeta_gmbh/
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties, and other factors, many of which are outside of OncoBeta's control, and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta® undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No pain reported during procedure.6
‡No direct skin contact or incisions.6
References
The EPIC-Skin Study (Efficacy of Personalised Irradiation with Rhenium-SCT for the Treatment of Non-Melanoma Skin Cancer) is designed to assess treatment efficacy and safety, as well as key patient-reported outcomes including quality of life, treatment comfort, and cosmetic results.1 The study is based on the clinically validated effect of the beta-emitter rhenium-188 in treating basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).2
Treatment data is available for 184 adult patients (with 254 lesions) across 7 study centres in Australia, Austria, Germany, the United Kingdom, and South Africa. All participants had histologically confirmed stage I or II NMSC. The median patient age was 70.3 years (range 27–95 years), and 53.3% of participants were male. Patients had one (71.2%), two (19.6%), or three (9.2%) lesions treated, which were located on the head & neck (11.8%), trunk (11.8%), lower limbs (11.8%), or upper limbs (9.1%).1
Treatment & Methodology
Rhenium-SCT was administered as a single 50-Gy topical dose of rhenium-188 embedded in a resin applied via adhesive film to the lesion site. Efficacy was assessed using modified RECIST criteria, at 12 months follow-up. Quality of life was assessed using the Skin Cancer Index (SCI) at baseline, 6 months, and 12 months. Treatment comfort was assessed using a patient questionnaire, whilst patient- and clinician-evaluated cosmetic outcomes were determined using a visual analogue scale (VAS) grading of 0-10 (0 = very poor; 10 = no wound detectable). Safety was assessed via treatment-emergent adverse events (TEAEs) and CTCAE (Common Terminology Criteria for Adverse Events) grading.
Key 12-Month Results:1
- Overall response rate: 97.3%
- Complete response rate: 94.1%
- Partial response rate: 3.2%
- Mean improvement in quality of life: +10.55 points from baseline
- Pain/discomfort during treatment: None reported
- Cosmetic outcomes: Favourable results reported by both patients and clinicians
- Most common toxicity (12-month): Grade 1 hypopigmentation (60.4%)
- No CTCAE toxicities above Grade 2 observed at 12 months
These findings confirm that Rhenium-SCT®, administered in a single session, delivers sustained high efficacy and improved quality of life, with excellent cosmetic outcomes and minimal adverse effects.
More on The PennZone
- AI Real Estate Company Quietly Building a National Powerhouse: reAlpha Tech Corp. (N A S D A Q: AIRE)
- Inkdnylon Expands National Uniform Embroidery Services
- Appliance EMT Expands Appliance Repair Services to Portland, OR and Vancouver, WA
- Next Week: The World's Best Young Pianists Arrive in Music City for the 2025 Nashville International Chopin Piano Competition
- Revenue Optics Builds Out Its Dedicated Sales Recruiting Firm with Strategic Addition of Christine Schafer
"Rhenium-SCT® has consistently demonstrated effectiveness and safety in prior studies," said Dr. Gerhard Dahlhoff, Medical Director at OncoBeta®. "This 12-month interim data further reinforces its value as a non-invasive, targeted treatment option for NMSC, particularly for patients seeking alternatives to surgery due to cosmetic or health-related concerns."
"The 12-month results from the EPIC-Skin Study represent a major milestone for OncoBeta®," added Shannon D. Brown III, CCO OncoBeta & Managing Director Europe. "These results support the efficacy, safety, and patient satisfaction associated with Rhenium-SCT®. These are the three foundational pillars of our patient-first vision, emphasising not only clinical outcomes, but also the patient experience in achieving them."
_
About the Rhenium-SCT® (Skin Cancer Therapy)
Non-melanoma skin cancer (NMSC) is the most common form of cancer in humans.3 The most common cause of NMSC is sun exposure, while other predisposing factors include genetic skin conditions and immunosuppressive diseases or treatments.4
The Rhenium-SCT is a painless*, non-invasive‡ therapy that provides aesthetic results, even in cases otherwise considered difficult to treat.1,5,6 The Rhenium-SCT utilises the radioisotope Rhenium-188 in an epidermal application with optimal properties for the treatment of NMSCs (non-melanoma skin cancers). The Rhenium-SCT is a precise, personalised therapy2 that is only applied to the area needed to treat without affecting the healthy tissue. The specially designed device ensures the Rhenium-SCT compound never comes in direct contact with the patient's skin and the application is safe and simple for the applying physician. Scar-free healing of the treated lesion area and the regeneration of healthy tissue occurs usually within a few weeks after treatment.6-8
About OncoBeta®
OncoBeta® with its headquarters located in Garching near Munich, Germany, is a privately held medical device company, specializing in the development and commercialization of state-of-the-art, innovative therapies. Since its foundation, OncoBeta® has concentrated its efforts on the development, regulatory approval(s) and commercialization of the epidermal radioisotope therapy Rhenium-SCT® (Skin Cancer Therapy), targeting non-melanoma skin cancers and keloid scars. OncoBeta® has perfected the customized application and device management system in conformity with all health, safety, and environmental protection regulatory standards.
More on The PennZone
- Hydrofast Elevates the Holiday Season: The C100 Countertop RO System Merges Smart Tech with Wellness for the Perfect Christmas Gift
- Melospeech Inc. Accepts Nomination for HealthTech Startup of the Year
- Flower City Tattoo Convention Draws Record Attendance in Rochester, NY
- KIKO NATION TOKEN (Official Release)
- Verb™ Presents Features Vanguard Personalized Indexing: Utilizing Advanced Tax-Loss Harvesting Technology
Find out more about the Rhenium-SCT® at www.oncobeta.com
Follow us on social media:
LinkedIn: https://www.linkedin.com/company/oncobeta-gmbh/
Facebook: https://www.facebook.com/oncobeta/
Instagram: https://www.instagram.com/oncobeta_gmbh/
Forward-looking statements
This announcement includes forward-looking statements that involve risks, uncertainties, and other factors, many of which are outside of OncoBeta's control, and which could cause actual results to differ materially from the results discussed in the forward-looking statements. Forward-looking statements include statements concerning OncoBeta's plans, objectives, goals, future events, performance and/or other information that is not historical information. All such forward-looking statements are expressly qualified by these cautionary statements and any other cautionary statements which may accompany the forward-looking statements. OncoBeta® undertakes no obligation to publicly update or revise forward-looking statements to reflect subsequent events or circumstances after the date made, except as required by law.
*No pain reported during procedure.6
‡No direct skin contact or incisions.6
References
- Cardaci G, et al. Adv Radiat Oncol. 2025;10(7):101802.
- Cipriani C, et al. J Dermatol Treat. 2022;33(2):969–975. Epub 22 Jul 2020.
- Ciążyńska M, et al. Sci Rep. 2021;11(1):4337.
- Cancer.net. Skin Cancer (Non-Melanoma): Risk Factors and Prevention. February 2022. https://www.cancer.net/cancer-types/skin-cancer-non-melanoma/risk-factors-and-prevention (accessed June 2025).
- Tietze JK, et al. Clin Nuc Med. 2023;48(10):869–876.
- Castellucci P, et al. Eur J Nucl Med Mol Imaging. 2021;48(5):1511–1521.
- Cipriani C, et al. Int J Nucl Med. 2017;114–112.
- Sedda AF, et al. Clin Exp Dermatol. 2008;33(6):745–749.
Source: OncoBeta GmbH
Filed Under: Health
0 Comments
Latest on The PennZone
- African American Genealogy Group Launches 2025 Raffle Fundraiser to Support Legacy Research
- Take Control of Your Color Matching with Boston Industrial Solutions' Newly Expanded Natron® UVPX Series Ink Colors
- "Dr. Vincent Michael Malfitano Expands Monterey–Sicily Cultural Diplomacy With Major International Media Engagement"
- Kaufman Development Breaks Ground on Detroit Micro Data Center, Expanding Its National AI Platform
- Cummings Graduate Institute for Behavioral Health Studies Celebrates New DBH Graduates
- $80M+ Backlog as Florida Statewide Contract, Federal Wins, and Strategic Alliance Fuel Next Phase of AI-Driven Cybersecurity Growth: Cycurion $CYCU
- High-Conviction CNS Disruptor Aiming to Transform Suicidal Depression, Ketamine Therapeutics, and TMS - Reaching Millions by 2030
- Top10Christmas.co.uk Releases the UK Christmas Toy Trends 2025 Report
- Talagat Business Academy Announces Joint Certificate Program With The University of Chicago Booth School of Business
- LocaXion and Asseco CEIT Announce First-to-Market RTLS-Driven Digital Twin Platform for Healthcare, Manufacturing, and Logistics
- Slotozilla Launches New Report on How AI Is Reshaping Careers and Society
- OKAVA Pharmaceuticals Announces First Cat Dosed in MEOW-1 Study of OKV-119, the World's First Clinical-Stage GLP-1 Weight-Loss Therapy for Pets
- Explosive Growth in U.S. Cryptocurrency Cloud Mining Sets The Stage for New Platform Launch with Daily Rewards in a Transparent Revenue-Share Model
- Qtex Cierra Ronda de $7 Millones para Estandarizar la Banca Transfronteriza en los Mercados Emergentes de Latinoamérica
- Ring in the Season with Free Holiday Jazz from The Jazz Sanctuary
- America's Most Festive Garages Wanted for Garage.com's 2025 Holiday Contest
- FDA Accepts ANDA for KETAFREE™ as Analyst Sets $34 Price Target for NRx Pharmaceuticals: (N A S D A Q : NRXP) NRx is Poised for a massive Breakthrough
- BEC Technologies Expands MX-220 5G Industrial Router Series for Edge Connectivity
- "Latino Leaders Speak: Personal Stories of Struggle and Triumph, Volume II" Documents the Truth About Latino Excellence and Impact on American Society
- Broadway Smile Boutique Unveils Modern Website for Enhanced Patient Experience