Trending...
- "They Said It Was Impossible": This Bottle Turns Any Freshwater Source Into Ice-Cold, Purified Drinking Water in Seconds
- Deep Learning Robotics (DLRob) Announces Pre-Launch of Zero-Teach and Teach-by-Demonstration Technology for Kitting Applications
- Excel Signworks Introduces Custom Lobby Signs to Help Pittsburgh Businesses Elevate First Impressions in 2026
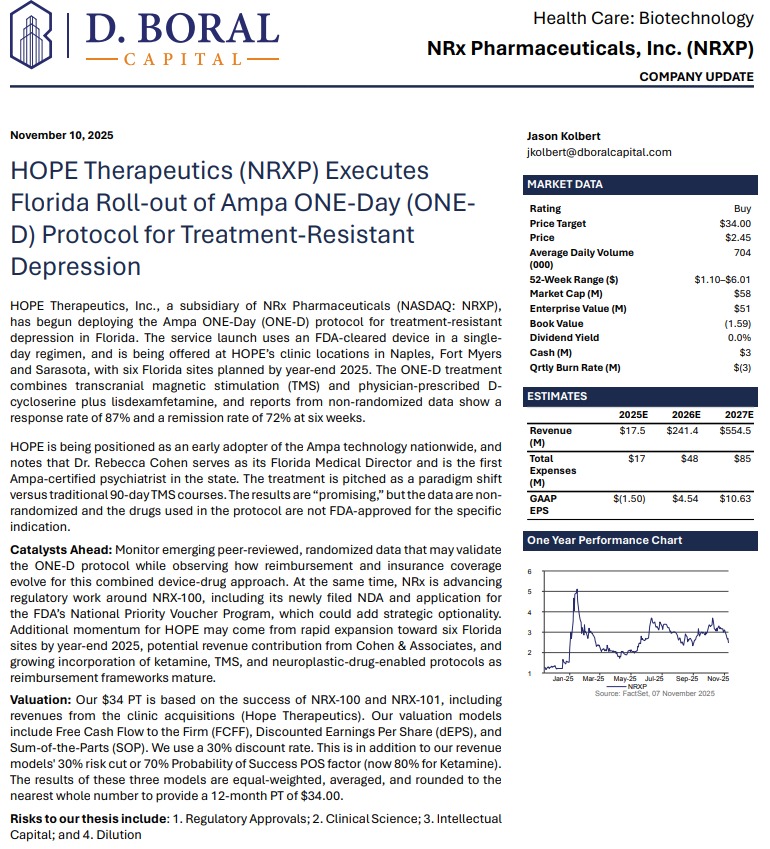
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP: FDA Alignment, 70,000-Patient Real-World Data, and a Debt-Free Balance Sheet Position NRXP for Transformational 2026 Catalysts
MIAMI - PennZone -- In a pivotal development that could redefine the treatment landscape for suicidal depression, NRx Pharmaceuticals (N A S D A Q: NRXP) has emerged from a high-level, in-person Type C meeting with the U.S. Food and Drug Administration with what management believes is a clear path toward New Drug Application (NDA) submission for NRX-100 (preservative-free ketamine)—supported by both prior clinical trials and an unprecedented real-world dataset exceeding 65,000 patients.
For investors, the implications are substantial: regulatory clarity, expanding indications, strategic partnerships, a strengthened balance sheet, and a global ketamine market estimated at $750 million annually—with no FDA-approved drug currently indicated specifically for suicidal ideation.
FDA Type C Meeting Signals Regulatory Momentum
NRXP's meeting was attended by leadership from the FDA Division of Psychiatry Products and the Center for Drug Evaluation and Research (CDER)—a strong signal of institutional engagement at the highest levels.
Key takeaways:
This broader indication meaningfully expands the addressable patient population and commercial potential.
NRX-100 has already received Fast Track Designation for the treatment of acute suicidality in depression and bipolar depression.
70,000 Patients: Real-World Data at Scale
NRXP licensed data from more than 70,000 U.S. patients treated with IV ketamine or nasal S-ketamine for depression and suicidal ideation.
Preliminary analysis of a 20,000-patient subset demonstrated:
Notably, there is currently no FDA-approved medication specifically indicated for suicidal ideation, with Electroconvulsive Therapy (ECT) remaining the primary intervention.
More on The PennZone
If FDA alignment continues, NRX-100 could become the first drug positioned specifically for this indication under Accelerated Approval.
KETAFREE™: First Preservative-Free Ketamine
NRXP has applied for the proprietary name KETAFREE™, designed to be the first preservative-free ketamine formulation submitted for approval in this setting.
Given existing clinical familiarity with ketamine, the transition from generic compounded products to an FDA-reviewed, preservative-free branded formulation presents a compelling commercialization thesis.
Dual Strategy: Drug Development + Clinic Network Expansion
In parallel, NRXP announced a joint offering with neurocare Group AG to create a nationwide network of integrated neuroplastic care clinics targeting:
The model integrates:
Recent publications demonstrate:
NRXP plans to integrate neurocare clinics with HOPE Therapeutics and leverage an installed base of 400+ Apollo® TMS machines nationwide, creating a scalable service revenue channel alongside drug commercialization.
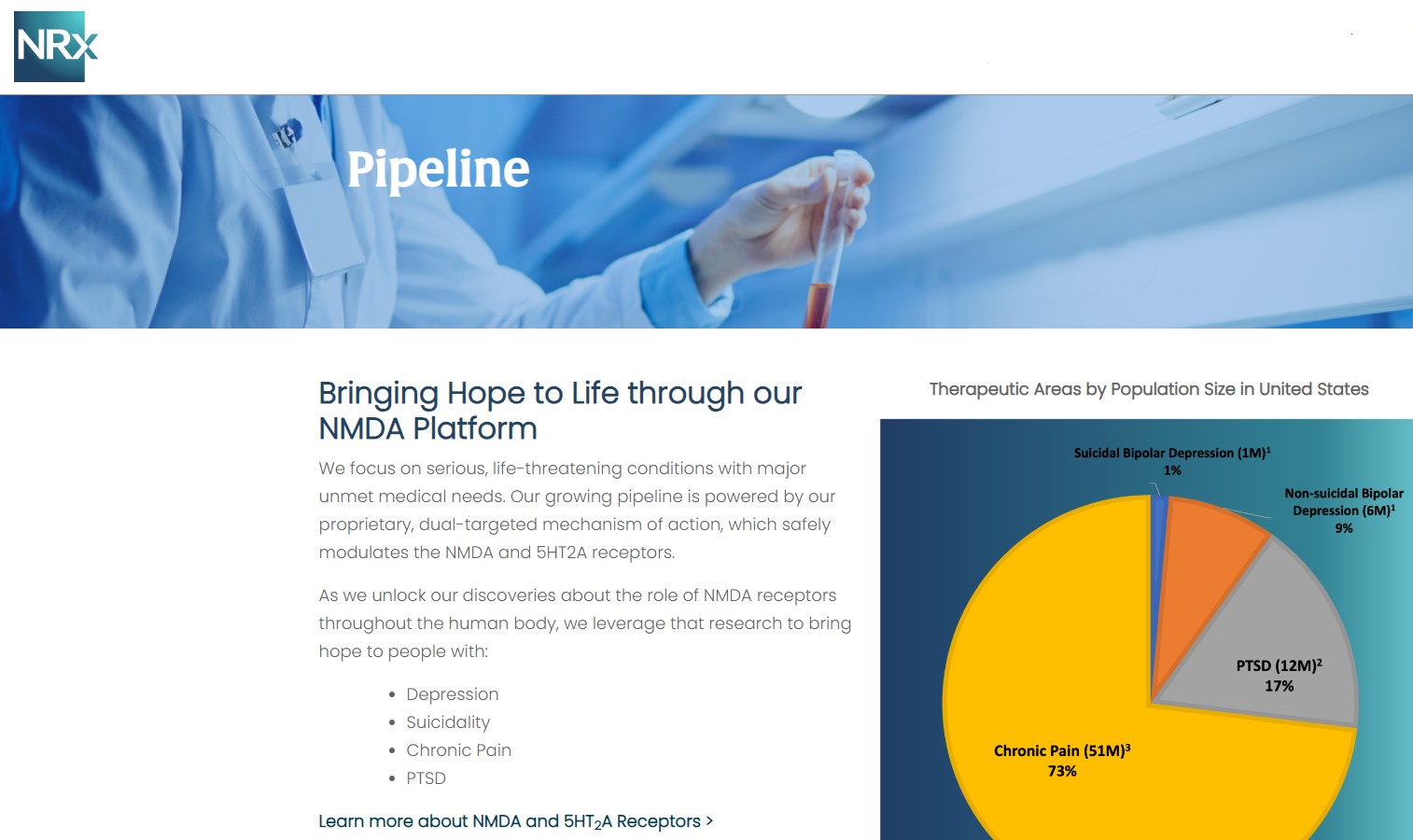
NRX-101: Breakthrough Therapy with Expanded Market Potential
Beyond NRX-100, NRXP is advancing NRX-101, an FDA-designated Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain.
Key attributes:
With projections suggesting 1 million Americans annually may receive TMS by 2030, NRX-101 could capture a significant share of a rapidly expanding neurostimulation market.
NRXP has partnered with Alvogen Pharmaceuticals for development and marketing of NRX-101 in suicidal bipolar depression.
Debt-Free Balance Sheet: Strategic Reset for Growth
More on The PennZone
In December, NRXP eliminated its remaining $5.4 million in balance sheet debt via equity conversion—with no warrants or toxic provisions.
This clean capital structure positions the company for:
Analyst Coverage: $34 Price Target
Investment firm D. Boral has issued a Buy rating with a $34 price target, citing regulatory progress, expanded indications, and commercial optionality.
For a company operating at the intersection of FDA reform, real-world evidence utilization, and neuroplastic treatment innovation, this represents a high-conviction thesis on both regulatory and market catalysts.
The Macro Opportunity
According to CDC data, more than 13 million Americans seriously consider suicide each year.
Yet there is no FDA-approved drug indicated specifically for suicidal ideation.
NRXP is positioning itself to:
This is not a single-asset story—it is a platform strategy targeting one of the largest and most urgent unmet needs in psychiatry.
Investment Thesis Snapshot
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
For investors, the implications are substantial: regulatory clarity, expanding indications, strategic partnerships, a strengthened balance sheet, and a global ketamine market estimated at $750 million annually—with no FDA-approved drug currently indicated specifically for suicidal ideation.
FDA Type C Meeting Signals Regulatory Momentum
NRXP's meeting was attended by leadership from the FDA Division of Psychiatry Products and the Center for Drug Evaluation and Research (CDER)—a strong signal of institutional engagement at the highest levels.
Key takeaways:
- Path to NDA based on existing adequate and well-controlled trial data
- Submission supported by 65,000+ patient Real-World Evidence (RWE) dataset
- No additional nonclinical studies required
- No bridging studies needed for preservative-free formulation
- Opportunity to seek broader indication: Treatment-Resistant Depression (TRD) with suicidality—not just acute suicidality
This broader indication meaningfully expands the addressable patient population and commercial potential.
NRX-100 has already received Fast Track Designation for the treatment of acute suicidality in depression and bipolar depression.
70,000 Patients: Real-World Data at Scale
NRXP licensed data from more than 70,000 U.S. patients treated with IV ketamine or nasal S-ketamine for depression and suicidal ideation.
Preliminary analysis of a 20,000-patient subset demonstrated:
- Rapid resolution of depression
- Rapid reduction in suicidal ideation
- Clinical outcomes consistent with prior randomized trials
- Favorable comparison to currently approved therapies
Notably, there is currently no FDA-approved medication specifically indicated for suicidal ideation, with Electroconvulsive Therapy (ECT) remaining the primary intervention.
More on The PennZone
- From Sleepless Nights to Sold-Out Drops: Catch Phrase Poet's First Year Redefining Motivational Urban Apparel
- Launching at 10AM Eastern: The Bottle That Chills and Purifies Any Water — No Ice Required
- How Specialized Game Development Services Are Powering the Next Wave of Interactive Entertainment
- Brain Drain Unlimited Strengthens Legal Advocacy with Advanced Training from Villanova University
- Don't Settle for a Lawyer Who Just Speaks Spanish. Demand One Who Understands Your Story
If FDA alignment continues, NRX-100 could become the first drug positioned specifically for this indication under Accelerated Approval.
KETAFREE™: First Preservative-Free Ketamine
NRXP has applied for the proprietary name KETAFREE™, designed to be the first preservative-free ketamine formulation submitted for approval in this setting.
Given existing clinical familiarity with ketamine, the transition from generic compounded products to an FDA-reviewed, preservative-free branded formulation presents a compelling commercialization thesis.
Dual Strategy: Drug Development + Clinic Network Expansion
In parallel, NRXP announced a joint offering with neurocare Group AG to create a nationwide network of integrated neuroplastic care clinics targeting:
- Depression
- PTSD
- Bipolar Depression
- Autism
- Traumatic Brain Injury
The model integrates:
- Transcranial Magnetic Stimulation (TMS)
- Ketamine and neuroplastic drugs
- Hyperbaric oxygen therapy
- Supportive psychotherapy
Recent publications demonstrate:
- 87% clinical response
- 72% remission
in treatment-resistant depression when combining TMS with neuroplastic therapies.
NRXP plans to integrate neurocare clinics with HOPE Therapeutics and leverage an installed base of 400+ Apollo® TMS machines nationwide, creating a scalable service revenue channel alongside drug commercialization.
NRX-101: Breakthrough Therapy with Expanded Market Potential
Beyond NRX-100, NRXP is advancing NRX-101, an FDA-designated Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain.
Key attributes:
- Composition-of-matter patent protection globally
- Combines D-cycloserine + low-dose lurasidone
- Designed to mitigate hallucination risk seen with DCS alone
- Newly amended IND to include augmentation of TMS
With projections suggesting 1 million Americans annually may receive TMS by 2030, NRX-101 could capture a significant share of a rapidly expanding neurostimulation market.
NRXP has partnered with Alvogen Pharmaceuticals for development and marketing of NRX-101 in suicidal bipolar depression.
Debt-Free Balance Sheet: Strategic Reset for Growth
More on The PennZone
- Dan Williams Promoted to Century Fasteners Corp. – General Manager, Operations
- Ski Johnson Inks Strategic Deals with Three Major Food Chain Brands
- NIL Club Advances Agent-Free NIL Model as Oversight Intensifies Across College Athletics
- Pallet Company Partners with Internet Marketing Company
- Atlanta Magazine Names Dr. Rashad Richey One of Atlanta's Most Influential Leaders in 2026 as the FIFA World Cup Approaches
In December, NRXP eliminated its remaining $5.4 million in balance sheet debt via equity conversion—with no warrants or toxic provisions.
This clean capital structure positions the company for:
- NDA filing preparation
- Clinic expansion
- Commercial scale-up
- Potential strategic partnerships
Analyst Coverage: $34 Price Target
Investment firm D. Boral has issued a Buy rating with a $34 price target, citing regulatory progress, expanded indications, and commercial optionality.
For a company operating at the intersection of FDA reform, real-world evidence utilization, and neuroplastic treatment innovation, this represents a high-conviction thesis on both regulatory and market catalysts.
The Macro Opportunity
According to CDC data, more than 13 million Americans seriously consider suicide each year.
Yet there is no FDA-approved drug indicated specifically for suicidal ideation.
NRXP is positioning itself to:
- Be first-to-label in suicidal depression
- Expand into broader treatment-resistant depression
- Integrate drug + device neuroplastic therapies
- Monetize both pharmaceutical and clinic service channels
This is not a single-asset story—it is a platform strategy targeting one of the largest and most urgent unmet needs in psychiatry.
Investment Thesis Snapshot
- ✅ Fast Track designation for NRX-100
- ✅ 70,000-patient real-world dataset
- ✅ Clear FDA path toward NDA
- ✅ Broader proposed indication
- ✅ Breakthrough Therapy designation (NRX-101)
- ✅ Expanding TMS augmentation indication
- ✅ Strategic partnership with neurocare
- ✅ Debt-free balance sheet
- ✅ Global ketamine market opportunity
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: CorporateAds
0 Comments
Latest on The PennZone
- Dave Aronberg Named 2026 John C. Randolph Award Recipient by Palm Beach Fellowship of Christians & Jews
- General Relativity Challenged by New Tension Discovered in Dark Siren Cosmology
- Burkentine Real Estate Group to Bring A New Community to Millersville, Pennsylvania
- Unseasonable Warmth Triggers Early Pest Season Along I-5 Corridor
- VIP Vacations Named Winner in 2026 WeddingWire Couples' Choice Awards®
- Colonial Nissan Champions Community Service and Trust Across the Greater Philadelphia Region
- Bug Busters Expands Service Footprint With New Carrollton, Georgia Branch
- Why KULR Could Be a Quiet Enabler of Space-Based Solar Power (SBSP) Over The Long Term: KULR Technology Group, Inc. (NY SE American: KULR)
- Why Finland Had No Choice But to Legalize Online Gambling
- High-Margin Energy & Digital Infrastructure Platform Created after Merger with Established BlockFuel Energy, Innovation Beverage Group (NAS DAQ: IBG)
- iFLO Pro Launches Its Groundbreaking iFLO Pro Mini At The 2026 AHR Expo In Las Vegas
- TL International Group Becomes First Global Operator to Fully Migrate to Pulsant's Dedicated Car Rental Cloud
- Diveroli Investment Group Files 13D in PetMed Express, Highlights Strategic Value, Asset Floor, and Multiple Takeover Pathways
- Excel Signworks Introduces Custom Lobby Signs to Help Pittsburgh Businesses Elevate First Impressions in 2026
- Deep Learning Robotics (DLRob) Announces Pre-Launch of Zero-Teach and Teach-by-Demonstration Technology for Kitting Applications
- The Quasar Dipole Phenomenon is likely just a complex systematics artifact
- The Rise of Comprehensive Home Water Treatment Systems
- Yazaki Innovations to Introduce First-Ever Prefabricated Home Wiring System to U.S. Residential Market in 2026
- Bisnar Chase Named 2026 Law Firm of the Year by Best Lawyers
- Ace Industries Welcomes Jack Polish as Controller