Trending...
- INVESTIGATION ALERT: Berger Montague PC Investigates Coinbase Global, Inc.'s Board Of Directors For Breach of Fiduciary Duties (NASDAQ: COIN)
- New Bethany Acquires Former Diocesan Headquarters to Expand Services
- Initial Order Received from Vietnamese Maritime Security and Defense Services for Advanced Video Compression Solution: RMX; Stock Symbol: RMXI
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Has $7.8 Million for Clinic Acquisitions and Purchase of Kadima Neuropsychiatry Institute as Treatment Model and Leading Investigative Site for Suicidal Depression / PTSD
MIAMI - PennZone -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Applied for New Commissioner's National Priority Vouchers (CNPV) for NRX-100 With Anticipated Decisions on Drug Approval by Year-End.
Application Under CNPV Program is Accretive to Already-Filed Abbreviated New Drug Application With Proprietary Formulation Under Priority Review Request.
Abbreviated New Drug Application Filed for Preservative-Free IV Ketamine.
Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Added to Previously Announced Term Sheet with a Strategic Investor, NRXP HOPE Funding of $10.3 Million is Planned in Near Term.
Agreement to Purchase Kadima Neuropsychiatry Institute Expected to Serve as Clinical Model for Treatment Offerings Nationwide.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research, Having Served as the Lead Site in Nearly All Major Trials in This Space.
Dr. David Feifel, Nationally Recognized Pioneer in Interventional Psychiatry to join as Chief Medical Innovation Officer.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Application to Uplist to NASDAQ Global Market from NASDAQ Capital Market.
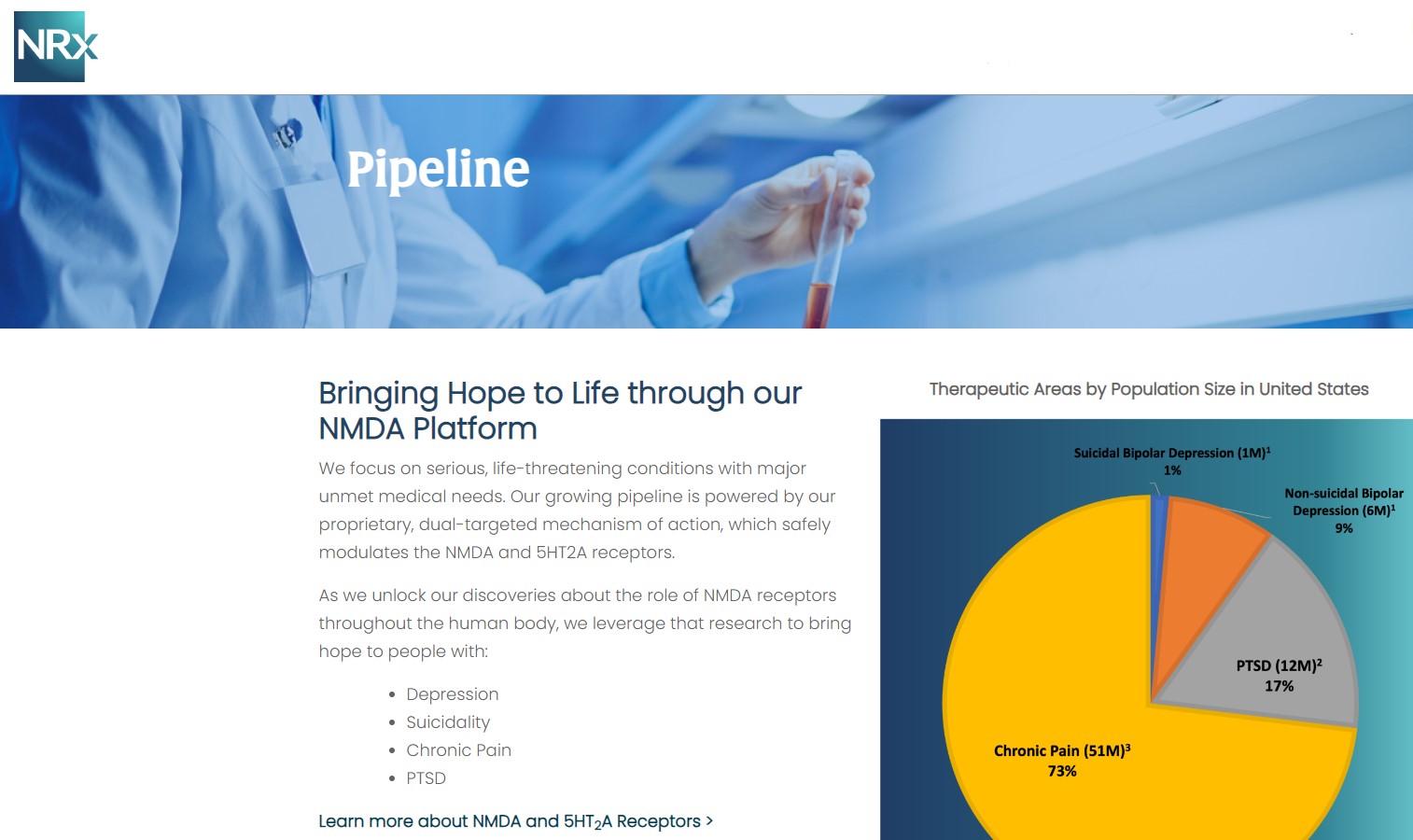
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
More on The PennZone
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
On June 17, 2025, FDA Commissioner Marty Makary, MD, MPH announced a new approval pathway, the Commissioner's National Priority Voucher (CPNV)1, for approval of drugs to enhance the health interests of Americans. Previously, on May 25 he identified psychedelic drugs for treatment of suicidal depression and PTSD as a national priority.2 The new voucher may be redeemed by drug developers to participate in a Commissioner-led program that shortens its review time from approximately 10-12 months to 1-2 months following a sponsor's final drug application submission.
To qualify for the CNPV, sponsors must submit the chemistry, manufacturing, controls (CMC) portion of the application and the draft labeling at least 60 days before submitting the final application. NRXP has already submitted the CMC portion for NRX-100 and received FDA feedback.
Concurrent with the CNPV process, NRXP is preparing a citizen petition to seek withdrawal of preservative-containing forms of ketamine, based on the toxicity associated with the benzethonium chloride preservative used in the historic formulation. NRXP has also filed a patent on its preservative-free manufacturing process. Approval of either the citizen petition, or the patent, would be expected to enable NRXP to gain market share in the current $750 million generic ketamine market that is forecast to reach $3-5 billion annually by 2033, in addition to a share of the market already established for ketamine products for treating depression.
Strategic Investor Relations Partnership with astr partners
On June 16th NRXP announced a strategic investor relations partnership with astr partners, a boutique investor relations and capital advisory firm focused on the life sciences sector.
astr partners will work with the NRXP and HOPE subsidiary executive teams to execute a comprehensive investor relations program that includes investor targeting, message development, earnings preparation, conference support, and proactive investor engagement.
Abbreviated New Drug Application (ANDA) for Preservative-Free IV Ketamine
On June 5th NRXP announced the transmission of its Abbreviated New Drug Application (ANDA) for electronic filing to the U.S. Food and Drug Administration (FDA) for NRX-100, its preservative-free IV ketamine formulation, for use in all existing approved indications such as anesthesia and pain management.
NRXP anticipates filing a citizen's petition with the FDA to remove benzethonium chloride, a known neurotoxic and cytotoxic substance, from presentations of ketamine intended for intravenous use. NRXP believes that the preservative-free feature of NRX-100 will be deemed of benefit to patients because of the known toxicity of closely related benzalkonium chloride in current drug products. Preservatives were originally added to sterile injectable products in an era when a single vial of medication was used to treat multiple patients, a practice no longer allowed in US hospitals. NRXP has demonstrated that there is no need for such preservatives to maintain stability and sterility in ketamine presentations intended for single-patient use. Should the citizen's petition be granted, all formulations of ketamine sold in the US could face a regulatory requirement to be preservative free.
More on The PennZone
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
HOPE's three initial acquisitions represent best-in-class clinics that offer neuroplastic treatments including NRXP ketamine and transcranial magnetic stimulation (TMS) to treat patients with severe depression, PTSD, and related central nervous system conditions. Neuroplastic treatments represent a rapidly emerging class of interventions that cause the growth of new connections (synapses) between brain cells that have been shown in multiple clinical trials to relieve symptoms of depression and suicidality. The FDA has approved TMS devices for a number of indications and has approved a nasal form of ketamine for treatment resistant depression. HOPE's parent company, NRXP, is currently applying to the FDA for approval of intravenous preservative-free ketamine to treat suicidal depression.
Agreement to Purchase Kadima Neuropsychiatry Institute; Foundational Acquisition for the NRXP HOPE Network of Interventional Psychiatry Clinics
On May 13th NRXP announced signing of a definitive agreement to purchase the Kadima Neuropsychiatry Institute. Kadima is expected to serve as the clinical model for treatment offerings in NRXP HOPE-acquired clinics and is expected to continue its role as a leading investigative site for research into neuroplastic therapies including psychedelic medications, transcranial magnetic stimulation (TMS), and hyperbaric therapy.
Kadima is one of California's flagship interventional psychiatry clinics and was among the first to bring ketamine treatment for central nervous system (CNS) disorders out of academic research settings and into clinical practice. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders. Further, Kadima and David Feifel, MD PhD, Founder and Medical Director of Kadima, have served as leaders in clinical trial work on emerging therapies in CNS for top companies in the industry. Importantly, the clinic is profitable and is forecast to continue growth going forward. Dr. Feifel will join NRXP HOPE as its first Chief Medical Innovation Officer upon closing of the acquisition.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Applied for New Commissioner's National Priority Vouchers (CNPV) for NRX-100 With Anticipated Decisions on Drug Approval by Year-End.
Application Under CNPV Program is Accretive to Already-Filed Abbreviated New Drug Application With Proprietary Formulation Under Priority Review Request.
Abbreviated New Drug Application Filed for Preservative-Free IV Ketamine.
Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Added to Previously Announced Term Sheet with a Strategic Investor, NRXP HOPE Funding of $10.3 Million is Planned in Near Term.
Agreement to Purchase Kadima Neuropsychiatry Institute Expected to Serve as Clinical Model for Treatment Offerings Nationwide.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research, Having Served as the Lead Site in Nearly All Major Trials in This Space.
Dr. David Feifel, Nationally Recognized Pioneer in Interventional Psychiatry to join as Chief Medical Innovation Officer.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Application to Uplist to NASDAQ Global Market from NASDAQ Capital Market.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
More on The PennZone
- The ITeam Ranked on Channel Partners 2025 MSP 501—Tech Industry's Most Prestigious List of Managed Service Providers Worldwide
- Lottery.com Inc. Secures $300 Million in Growth Capital, Confirms Nasdaq Compliance & Acquires UAE Sports Incubator Amid High-Profile Brand Exposure
- Jason Daria Installed as President of Philadelphia Trial Lawyers Association
- NUFABRX awarded Therapeutic Compression agreement with Premier, Inc
- Luxurious Estate in Keenes Pointe Hits the Market at $2.9 Million
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
On June 17, 2025, FDA Commissioner Marty Makary, MD, MPH announced a new approval pathway, the Commissioner's National Priority Voucher (CPNV)1, for approval of drugs to enhance the health interests of Americans. Previously, on May 25 he identified psychedelic drugs for treatment of suicidal depression and PTSD as a national priority.2 The new voucher may be redeemed by drug developers to participate in a Commissioner-led program that shortens its review time from approximately 10-12 months to 1-2 months following a sponsor's final drug application submission.
To qualify for the CNPV, sponsors must submit the chemistry, manufacturing, controls (CMC) portion of the application and the draft labeling at least 60 days before submitting the final application. NRXP has already submitted the CMC portion for NRX-100 and received FDA feedback.
Concurrent with the CNPV process, NRXP is preparing a citizen petition to seek withdrawal of preservative-containing forms of ketamine, based on the toxicity associated with the benzethonium chloride preservative used in the historic formulation. NRXP has also filed a patent on its preservative-free manufacturing process. Approval of either the citizen petition, or the patent, would be expected to enable NRXP to gain market share in the current $750 million generic ketamine market that is forecast to reach $3-5 billion annually by 2033, in addition to a share of the market already established for ketamine products for treating depression.
Strategic Investor Relations Partnership with astr partners
On June 16th NRXP announced a strategic investor relations partnership with astr partners, a boutique investor relations and capital advisory firm focused on the life sciences sector.
astr partners will work with the NRXP and HOPE subsidiary executive teams to execute a comprehensive investor relations program that includes investor targeting, message development, earnings preparation, conference support, and proactive investor engagement.
Abbreviated New Drug Application (ANDA) for Preservative-Free IV Ketamine
On June 5th NRXP announced the transmission of its Abbreviated New Drug Application (ANDA) for electronic filing to the U.S. Food and Drug Administration (FDA) for NRX-100, its preservative-free IV ketamine formulation, for use in all existing approved indications such as anesthesia and pain management.
NRXP anticipates filing a citizen's petition with the FDA to remove benzethonium chloride, a known neurotoxic and cytotoxic substance, from presentations of ketamine intended for intravenous use. NRXP believes that the preservative-free feature of NRX-100 will be deemed of benefit to patients because of the known toxicity of closely related benzalkonium chloride in current drug products. Preservatives were originally added to sterile injectable products in an era when a single vial of medication was used to treat multiple patients, a practice no longer allowed in US hospitals. NRXP has demonstrated that there is no need for such preservatives to maintain stability and sterility in ketamine presentations intended for single-patient use. Should the citizen's petition be granted, all formulations of ketamine sold in the US could face a regulatory requirement to be preservative free.
More on The PennZone
- CredHub and ManageAmerica Partner to Empower Residents Through Seamless Rent Reporting Integration
- TKSoftware Inc. Unveils ICONIC Pro: The All‑In‑One Medical Billing & Clearinghouse Solution
- Baker Rights and Coercive Psychiatry: The Citizens Commission on Human Rights of Florida Hosts Monthly Mental Health Law and Human Rights Seminars
- Venardi Zurada LLP Offers Legal Support to Families After Deadly Lake Tahoe Boat Capsizing
- Elevated Healing Treatment Centers: Redefining Mental Health Care with Compassionate, Evidence-Based, and Accessible Services
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
HOPE's three initial acquisitions represent best-in-class clinics that offer neuroplastic treatments including NRXP ketamine and transcranial magnetic stimulation (TMS) to treat patients with severe depression, PTSD, and related central nervous system conditions. Neuroplastic treatments represent a rapidly emerging class of interventions that cause the growth of new connections (synapses) between brain cells that have been shown in multiple clinical trials to relieve symptoms of depression and suicidality. The FDA has approved TMS devices for a number of indications and has approved a nasal form of ketamine for treatment resistant depression. HOPE's parent company, NRXP, is currently applying to the FDA for approval of intravenous preservative-free ketamine to treat suicidal depression.
Agreement to Purchase Kadima Neuropsychiatry Institute; Foundational Acquisition for the NRXP HOPE Network of Interventional Psychiatry Clinics
On May 13th NRXP announced signing of a definitive agreement to purchase the Kadima Neuropsychiatry Institute. Kadima is expected to serve as the clinical model for treatment offerings in NRXP HOPE-acquired clinics and is expected to continue its role as a leading investigative site for research into neuroplastic therapies including psychedelic medications, transcranial magnetic stimulation (TMS), and hyperbaric therapy.
Kadima is one of California's flagship interventional psychiatry clinics and was among the first to bring ketamine treatment for central nervous system (CNS) disorders out of academic research settings and into clinical practice. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders. Further, Kadima and David Feifel, MD PhD, Founder and Medical Director of Kadima, have served as leaders in clinical trial work on emerging therapies in CNS for top companies in the industry. Importantly, the clinic is profitable and is forecast to continue growth going forward. Dr. Feifel will join NRXP HOPE as its first Chief Medical Innovation Officer upon closing of the acquisition.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on The PennZone
- Nina Creative Designs Redefines Creative Solutions Under Visionary Leadership of CEO Sonny Parker
- Red Carpet Screening of "Ghosted" Featuring Landon Brittain at the Samuel Goldwyn Theatre
- Anti-war groups protest vs US-NATO actions in Gaza, Iran, the Global South
- Dr. Jacqueline West Honored as Best Dentist in JAX by Folio Weekly
- Exciting News: Pivotal Health Solutions Acquires Revolutionary Portable Parallel Bars
- Pennsylvania Listings and Home Prices Continue Upward Trend
- Miboxer Achieved New Goal in GILE 2025
- Jeopardy!'s Ken Jennings Headlines National Mensa Event
- Mensa Foundation Prize Awarded to Neuroscientist-Pianist
- New book, "High-Tech Heroes," redefines billionaire as someone who improves a billion lives
- Durex Products Wire Cloth Screen Media: Engineered for Maximum Performance and Durability
- Graybar Names Larry Smith District Vice President in Pittsburgh
- OPRAH.COM Featured Award-Winning Novel AS FAR AS YOU GO BEFORE YOU HAVE TO COME BACK now Available as Audiobook
- How Kallie Boxell Helps Texas Companies Solve the Talent Equation
- KeysCaribbean Vacation Home Rentals Offers Last-Minute Booking Discount of 15 Percent
- purelyIV Blog Named One of the Top 45 IV Therapy Blogs by Feedspot
- purelyIV Launches Mobile Iron Infusion Therapy for Patients with Iron Deficiency Anemia
- Smile Makers Dental Care Introduces FP1: East Bay's First Robotic-Assisted Full-Arch Implant Solution for Natural, Fixed Smiles
- DCAS College opens new Representative Office in Malaysian Capital Kuala Lumpur
- GMO Miner: Creating a simple, efficient and reliable new cloud mining experience