Trending...
- K-Drama Tours Expands Into K-Pop Experiences with New "K-Pop Demon Hunters" Tour in Seoul
- SJC Ventures closes on property and announces expansive lineup coming to Broadcast District
- Building A Business Website That Works In 2025
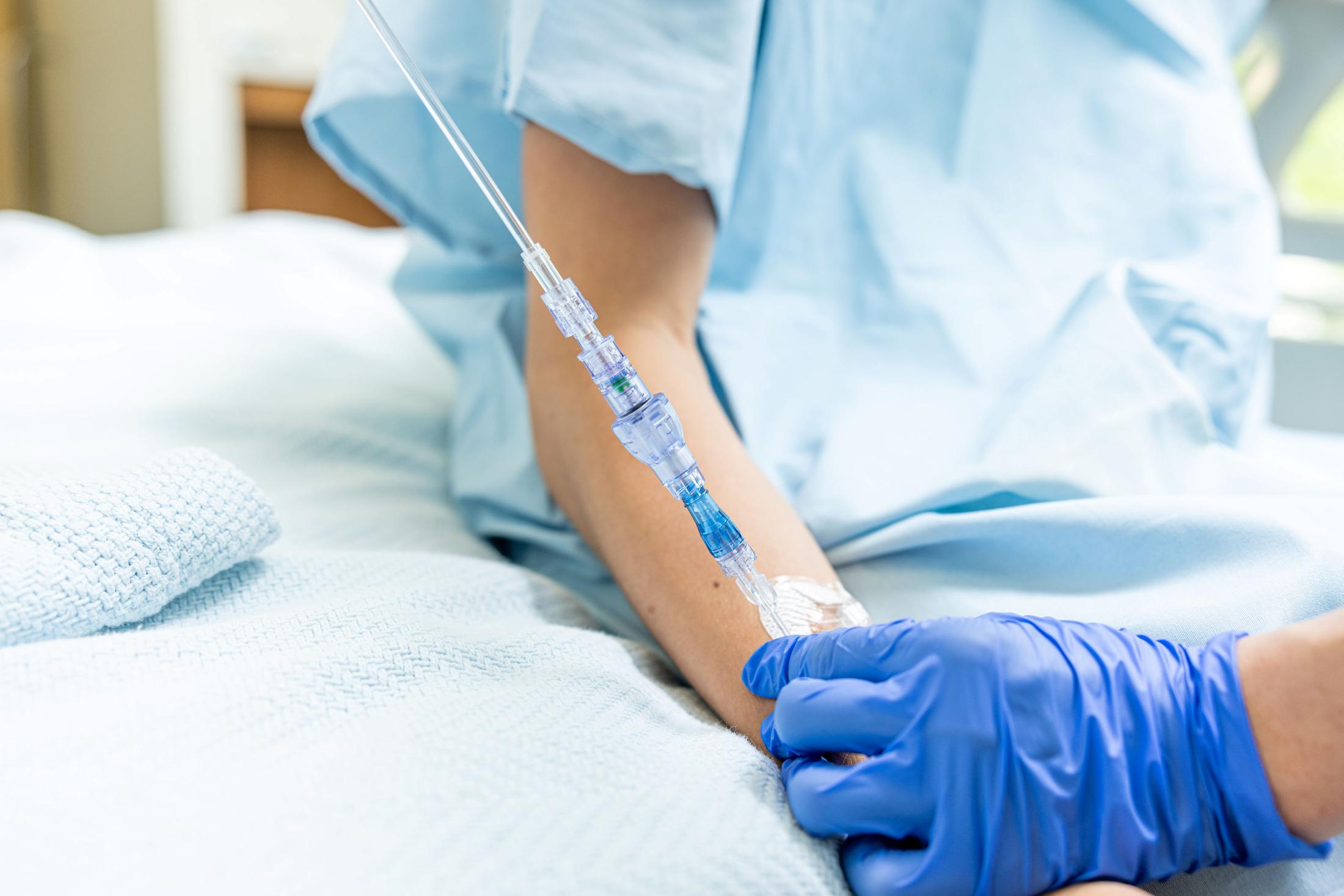
FAYETTEVILLE, Ark. - PennZone -- Lineus Medical has obtained CE Mark certification for its flagship product, SafeBreak® Vascular, the only breakaway device for IV lines clinically proven to reduce multiple IV complications.1 With this certification, SafeBreak Vascular is now cleared for use across the European Union. This approval expands SafeBreak Vascular's clearance from 5 countries to 32, spanning across more than three continents. Lineus Medical is actively partnering with distributors across Europe to expand hospital access to SafeBreak worldwide.
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on The PennZone
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on The PennZone
- DGiTK – Digital Technologies, LLC Announces Groundbreaking Partnership with Hyperscale Compute Partner to Revolutionize Data Sovereignty in the U.S
- Delirious Comedy Club Expands to Two Rooms and Secures Google's #1 Rated Comedy Club in Las Vegas
- SPOZZ, the Community-Owned Direct-to-Fan Music Ecosystem, adds "BEATS" — a Creator-to-Creator Marketplace
- Family-Owned Dave's Auto Services Celebrates 25 Years of Excellence in Boyertown
- DAECO Painting Sets the Gold Standard for High-End Interior Painting Services in Denver, CO
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
Source: Lineus Medical
Filed Under: Health
0 Comments
Latest on The PennZone
- Vesica Health Granted PLA Billing Code for AssureMDx
- Footasylum and invent.ai Optimize Inventory to Boost Revenue
- Newest Mako Smartrobotics™ System Used for First Time in the State Of Illinois for Total Joint Replacement Surgery
- $20 Million Annualized Revenue Projected from 20+ Acquisitions and Scaling of Top Quality Dental Labs Across Florida: Standard Dental Labs $TUTH
- Grok Wrote a Direct Message to Elon Musk Discussing Netverse & Phinge CEOs Challenge to Live Debate & Added "it'd be epic to see you two hash it out"
- Assent Recognized as a Leader in First-Ever Product Compliance Green Quadrant
- Tina Glasneck Launches New Romantasy, A Dragon's Queen, Blending Dragons, Fae Courts, and Forbidden Love
- Announcing the "Utsunomiya Gyoza Festival 2025" to be held November 1 and 2 in Utsunomiya City, Tochigi Pref, Japan
- Sine Nomine Associates: OpenAFS Google Summer of Code projects have been completed
- HuskyTail Digital Marketing Rings in Fall with Free SEO Audits for Local Businesses
- Bright Hope Baptist Church Fall Bazaar
- David White DDS Advances Implant Dentistry with New Technology Acquisition
- Final Countdown: The OpenSSL Conference 2025 Begins in One Week
- New Frontier Aerospace Appoints Industry Veteran Rich Pournelle as Director of Business Development
- AI's Urgent Energy Requirements Won't Be Solved By Trillions Of Dollars. Phinge's Patented App-Less Netverse Platform & Hardware Will Reduce This Need
- $750 Million Market Projected to Reach $3.35 Billion; Huge Opportunity for Superior Preservative-Free Ketamine Drug Treating Suicidal Depression $NRXP
- €6.4 Million in Contracts Across Multiple Countries; Smart City Developer; U.S. Expansion, and Announces Strategic Drone Tech Partnership; $AFFU
- CRYPTOCURRENCY: Lucrumia Exchange Platform Addresses Italian Traders' Growing Demand for Secure Digital Asset Trading
- NIUFO Launches Secure Trading Platform for Italian Market Seeking Stability After 20% User Decline