Trending...
- UK Financial Ltd Executes Compliance Tasks Ahead Of First-Ever ERC-3643 Exchange-Traded Token, SMCAT & Sets Date For Online Investor Governance Vote
- Phinge Founder & CEO Robert DeMaio Ranked #1 Globally on Crunchbase, Continues to Convert Previous Debt Owed to Him by Phinge into Convertible Notes
- Neurosurgeon Chengyuan Wu, MD, MSBmE, Joins the Actuated Medical Advisory Board
$NRXP Has Manufactured Multiple Commercial Lots of NRX-100 and KETAFREE™ with a Shelf Life of Three Years.
MIAMI - PennZone -- NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is rapidly emerging as one of the most compelling growth stories in mental-health therapeutics. With three revenue-generating treatment facilities now operating in Florida—and six expected by year-end—the company is entering 2026 with accelerating clinical operations, expanding market share, and advancing two FDA-directed regulatory pathways for ketamine-based therapeutics aimed at suicidal depression, one of the largest unmet needs in mental health.
Backed by strong clinical data, FDA Fast Track designations, and third-party revenue projections, NRXP appears positioned to capitalize on a rapidly expanding U.S. ketamine market currently estimated at $750 million, projected to reach $3.35 billion globally by 2034.
Adding to investor enthusiasm, analyst D. Boral issued a Buy rating and a $34 price target, citing NRXP's accelerating execution and diversified commercialization strategy.
A Mission Targeting One of America's Most Pressing Health Crises
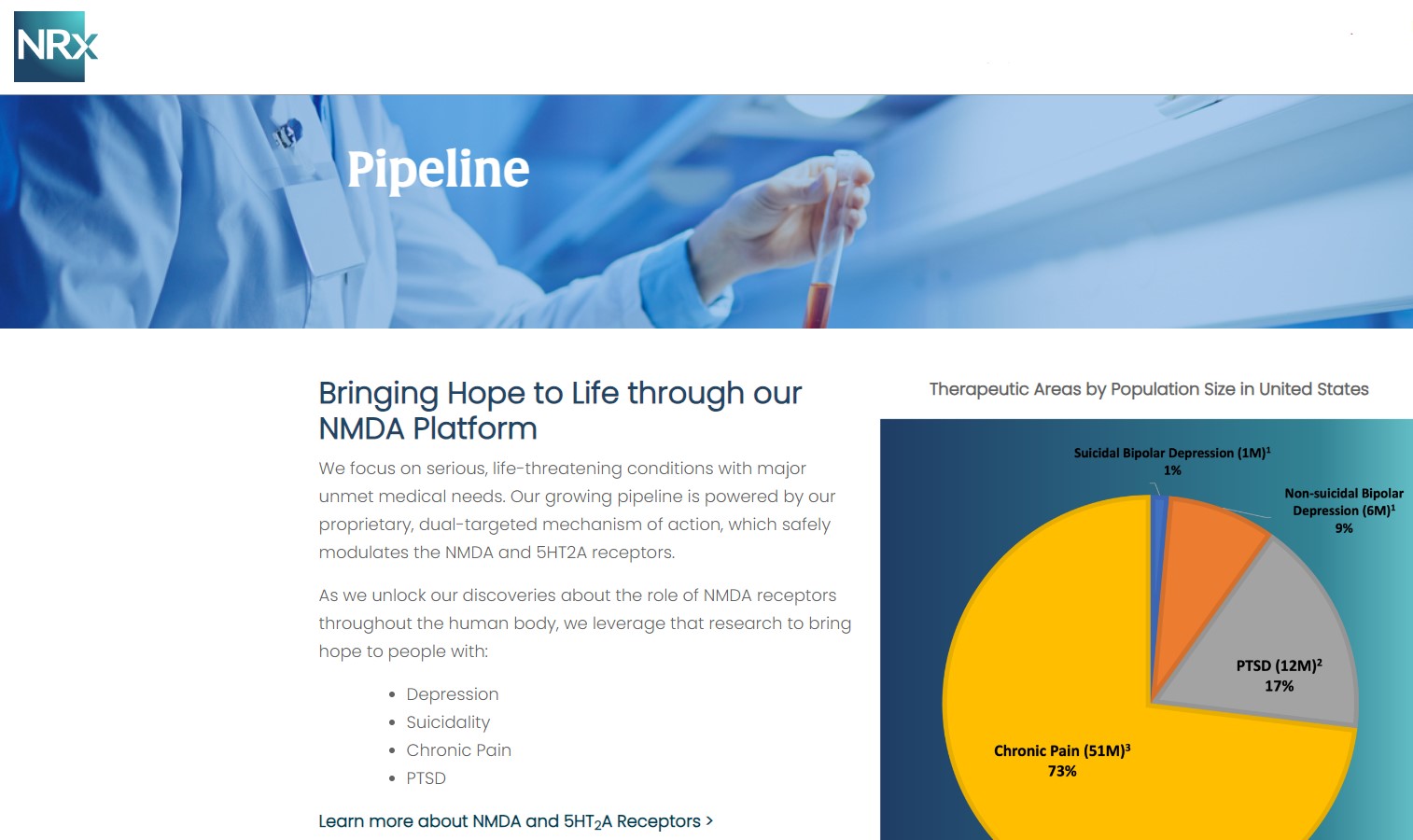
More than 13 million Americans seriously consider suicide each year (CDC). NRXP is developing next-generation therapeutics aimed directly at this crisis through its NMDA-based drug platform:
Key Pipeline Assets
NRXP is partnered with Alvogen Pharmaceuticals for the development and commercialization of NRX-101.
More on The PennZone
A Dual-Path Strategy for NRX-100: Innovative and Generic Market Entry
NRXP's regulatory strategy is unique: it is simultaneously pursuing both an innovative NDA pathway and a generic ANDA pathway for NRX-100 and KETAFREE™.
This creates two powerful value drivers:
1. Innovative NDA for Suicidal Depression
2. ANDA for KETAFREE™ (Generic Ketamine)
With commercial lots already manufactured, NRXP is positioned for a swift commercial rollout upon approval.
HOPE Clinics: Expanding Revenue Footprint Across Florida
NRXP's HOPE Therapeutics subsidiary is advancing a scalable clinic model for ketamine and advanced TMS-based interventions.
Current & Expected Expansion
These facilities support:
Management expects increased revenue contributions from clinical operations through 2026.
Breakthrough ONE-D: First-in-Florida Deployment with Ampa Health
In November, NRXP launched patient treatment using Ampa Health's ONE-D protocol—a groundbreaking approach reporting:
ONE-D combines:
ONE-D is being deployed at HOPE clinics in Sarasota, Naples, and Fort Myers, with additional locations coming online in 2025.
More on The PennZone
This marks a major competitive differentiator for NRXP in the mental-health treatment sector.
Financial Position: Capital Secured Through July 2026
NRXP reported securing sufficient operating capital to support its drug development programs through July 2026, complementing increasing clinical revenues.
Third-quarter results (released November 17, 2025) highlighted:
The full Q3 update and webcast are available on the NRXP investor relations site.
High-Value Licensing Opportunity
NRXP has accepted non-binding potential terms from an international pharmaceutical partner for the licensing and distribution of NRX-100. Terms include:
If finalized, this could represent a transformative revenue opportunity for the company.
Investment Outlook
With:
NRx Pharmaceuticals is attracting increased attention from both institutional and retail investors.
The recent $34 price target from D. Boral underscores growing confidence in NRXP's ability to execute on a scalable, high-margin strategy targeting one of the most underserved and urgent areas of mental health.
For More Information
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Chief Business Officer: Matthew Duffy
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Backed by strong clinical data, FDA Fast Track designations, and third-party revenue projections, NRXP appears positioned to capitalize on a rapidly expanding U.S. ketamine market currently estimated at $750 million, projected to reach $3.35 billion globally by 2034.
Adding to investor enthusiasm, analyst D. Boral issued a Buy rating and a $34 price target, citing NRXP's accelerating execution and diversified commercialization strategy.
A Mission Targeting One of America's Most Pressing Health Crises
More than 13 million Americans seriously consider suicide each year (CDC). NRXP is developing next-generation therapeutics aimed directly at this crisis through its NMDA-based drug platform:
Key Pipeline Assets
- NRX-100 (IV Ketamine):
- FDA Fast Track designation for suicidal ideation in depression, including bipolar depression.
- Multiple commercial lots produced with three-year shelf life stability.
- NDA submission expected in Q4 2025, supported by real-world patient data from more than 60,000 IV ketamine cases.
- KETAFREE™ (preservative-free ketamine):
- Pursuing approval via an ANDA (generic) pathway.
- Recently received supportive FDA correspondence confirming no major deficiencies and on track for a Q2 2026 GDUFA date.
- Addresses a ketamine market worth ~$750 million annually.
- NRX-101 (D-cycloserine + lurasidone):
- FDA-designated Investigational Breakthrough Therapy.
- Targets suicidal treatment-resistant bipolar depression, chronic pain, and potential utility in complicated UTIs.
- New real-world data suggest its active ingredient doubles the effectiveness of TMS—a potential major indication expansion.
NRXP is partnered with Alvogen Pharmaceuticals for the development and commercialization of NRX-101.
More on The PennZone
- Lacy Hendricks Earns Prestigious MPM® Designation from NARPM®
- Walmart $WMT and COSTCO.COM $COST Distribution as SonicShieldX™ Platform Sets the Stage for Accelerated Growth in 2026: AXIL Brands (N Y S E: AXIL)
- AI-Driven Drug Development with Publication of New Bioinformatics Whitepaper for BullFrog AI: $BFRG Strengthens Its Position in AI Drug Development
- IQSTEL Enters 2026 from a Position of Strength Following Transformational Year Marked by N A S D A Q Uplisting, Record Revenue and First-Ever
- Are You Hiring The Right Heater Repair Company in Philly?
A Dual-Path Strategy for NRX-100: Innovative and Generic Market Entry
NRXP's regulatory strategy is unique: it is simultaneously pursuing both an innovative NDA pathway and a generic ANDA pathway for NRX-100 and KETAFREE™.
This creates two powerful value drivers:
1. Innovative NDA for Suicidal Depression
- Expected completion: Q4 2025.
- Includes comparative real-world data showing IV ketamine may have faster onset and greater effect than intranasal S-ketamine.
- NRXP has applied for a Commissioner's National Priority Voucher, which could accelerate FDA review.
2. ANDA for KETAFREE™ (Generic Ketamine)
- Re-filed after FDA approved NRXP's Suitability Petition for its preservative-free formulation.
- Supportive FDA feedback in November 2025 indicated no significant deficiencies.
- A Citizen Petition filed to remove toxic preservative benzethonium chloride from ketamine products could reshape the competitive landscape.
With commercial lots already manufactured, NRXP is positioned for a swift commercial rollout upon approval.
HOPE Clinics: Expanding Revenue Footprint Across Florida
NRXP's HOPE Therapeutics subsidiary is advancing a scalable clinic model for ketamine and advanced TMS-based interventions.
Current & Expected Expansion
- 3 operational facilities in Florida
- 6 additional facilities planned by year-end
These facilities support:
- NRX-100 Expanded Access Program
- Ketamine-based clinical services
- ONE-D single-day TMS depression treatments
- Veterans, first responders, and active-duty military populations
Management expects increased revenue contributions from clinical operations through 2026.
Breakthrough ONE-D: First-in-Florida Deployment with Ampa Health
In November, NRXP launched patient treatment using Ampa Health's ONE-D protocol—a groundbreaking approach reporting:
- 87% response rate
- 72% remission
- Achieved in one day, instead of traditional 90-day TMS schedules
ONE-D combines:
- Ampa's FDA-cleared TMS device
- D-cycloserine (NRX-101 active ingredient)
- Lisdexamfetamine
ONE-D is being deployed at HOPE clinics in Sarasota, Naples, and Fort Myers, with additional locations coming online in 2025.
More on The PennZone
- Neurosurgeon Chengyuan Wu, MD, MSBmE, Joins the Actuated Medical Advisory Board
- Appliance EMT Expands Professional Appliance Repair Services to Hartford, Connecticut
- Java Holdings LLC Acquires +Peptide, Expanding Portfolio Across Coffee, Science, and Functional Nutrition
- OneSolution® Expands to Orlando with New Altamonte Springs Implant Center
- Indian Peaks Veterinary Hospital Launches Updated Dental Services Page for Boulder Pet Owners
This marks a major competitive differentiator for NRXP in the mental-health treatment sector.
Financial Position: Capital Secured Through July 2026
NRXP reported securing sufficient operating capital to support its drug development programs through July 2026, complementing increasing clinical revenues.
Third-quarter results (released November 17, 2025) highlighted:
- Progress across all clinical and regulatory objectives
- Expanded Fast Track designation for NRX-100
- EU and NIH data enhancing regulatory strategy
- Growth of the HOPE facility platform
- Enhanced IP and formulation positioning
The full Q3 update and webcast are available on the NRXP investor relations site.
High-Value Licensing Opportunity
NRXP has accepted non-binding potential terms from an international pharmaceutical partner for the licensing and distribution of NRX-100. Terms include:
- Over $300 million in milestone payments
- Tiered double-digit royalties
If finalized, this could represent a transformative revenue opportunity for the company.
Investment Outlook
With:
- Multiple clinical catalysts approaching
- Two regulatory pathways advancing for NRX-100
- FDA Breakthrough Therapy designations
- National leadership in cutting-edge TMS + D-cycloserine therapy
- Increasing real-world data support
- A growing network of revenue-producing treatment centers
- Newly manufactured commercial drug supply
- And a strong capital runway
NRx Pharmaceuticals is attracting increased attention from both institutional and retail investors.
The recent $34 price target from D. Boral underscores growing confidence in NRXP's ability to execute on a scalable, high-margin strategy targeting one of the most underserved and urgent areas of mental health.
For More Information
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Chief Business Officer: Matthew Duffy
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
Filed Under: Health
0 Comments
Latest on The PennZone
- Genuine Hospitality, LLC Selected to Operate Hilton Garden Inn Birmingham SE / Liberty Park
- Together We Dance Foundation Announces Transformational Support from NAC Have a Heart Foundation
- Harry Hayman Celebrates Years of WHYY Coverage, Partnership & Shared Commitment to Philadelphia
- Documentary "Prescription for Violence: Psychiatry's Deadly Side Effects" Premieres, Exposes Link Between Psychiatric Drugs and Acts of Mass Violence
- Stockwell Elastomerics expands micro molding capabilities
- Price Improvement on Luxurious Lāna'i Townhome with Stunning Ocean Views
- Nextvisit Co-Founder Ryan Yannelli Identifies Six Critical Factors for Behavioral Health Providers Evaluating AI Scribes in 2026
- CredHub and Real Property Management Join Forces to Empower Franchise Owners with Rental Payment Credit Reporting Solutions
- Leimert Park Announces Weeklong Kwanzaa Festival & Kwanzaa Parade Celebrating Black History, Culture, and Community
- Schmuck Lumber Ace Hardware Opens New Greenhouse Addition
- Renowned Alternative Medicine Specialist Dr. Sebi and His African Bio Mineral Balance Therapy Are the Focus of New Book
- Psychiatric Drug Damage Ignored for Decades; CCHR Demands Federal Action
- Why Millions Are Losing Sexual Sensation, And Why It's Not Age, Hormones, or Desire
- Justin Jeansonne An Emerging Country Singer-Songwriter Music Fans Have Been Waiting For…a True Maverick
- Russellville Huntington Learning Center Expands Access to Literacy Support; Approved Provider Under Arkansas Department of Education
- UK Financial Ltd Launches U.S. Operations Following Delaware Approval
- Pinealage: the app that turns strangers into meditation companions — in crowdfunding phase
- Top Tips for Hiring HVAC Contractors in Philadelphia
- Harry Hayman of Feed Philly Coalition Proudly Supports Sharing Excess' Holiday Food Rescue — Bri
- Virtual Pizza Academy Announces the Return of Two Acclaimed Live Classes in 2026: