Trending...
- Price Improvement on Luxurious Lāna'i Townhome with Stunning Ocean Views
- Russellville Huntington Learning Center Expands Access to Literacy Support; Approved Provider Under Arkansas Department of Education
- Justin Jeansonne An Emerging Country Singer-Songwriter Music Fans Have Been Waiting For…a True Maverick
H.C. Wainwright Initiates Coverage with $34 Price Target, Citing Paradigm Shift in Depression Treatment. NRx Pharmaceuticals (N A S D A Q: NRXP) $NRXP
MIAMI - PennZone -- NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP), a clinical-stage biopharmaceutical company pioneering NMDA-based therapeutics for central nervous system disorders, is emerging as a potential leader in the next generation of depression and suicidality treatments. With its innovative ONE-D (One Day Depression) protocol now launched in Florida and a $40 price target set by H.C. Wainwright, NRXP appears positioned for transformational growth in a rapidly expanding $3.35 billion global market.
A Major Step Forward: ONE-D Depression Treatment Launches in Florida
NRx Pharmaceuticals has initiated the first-in-Florida rollout of the ONE-D (One Day) Depression Treatment, in partnership with Ampa Health. The innovative protocol has shown breakthrough potential in achieving remission from treatment-resistant depression within a single day—an unprecedented leap forward for millions suffering from chronic depression.
Unlike traditional transcranial magnetic stimulation (TMS) therapies requiring up to 90 sessions over three months, the ONE-D approach combines a single-day TMS treatment with physician-prescribed compounds, including D-cycloserine, an active component of NRx's investigational NRX-101, and lisdexamfetamine. Early clinical data show an 87% response rate and 72% remission rate in nonrandomized studies.
The Ampa device is now available across NRXP HOPE Clinics in Sarasota, Naples, and Fort Myers, Florida—with six total locations expected by the end of 2025. This initiative marks one of the first national deployments of the Ampa ONE-D protocol.
More on The PennZone
Breakthrough Therapeutic Portfolio: NRX-101 and KETAFREE™
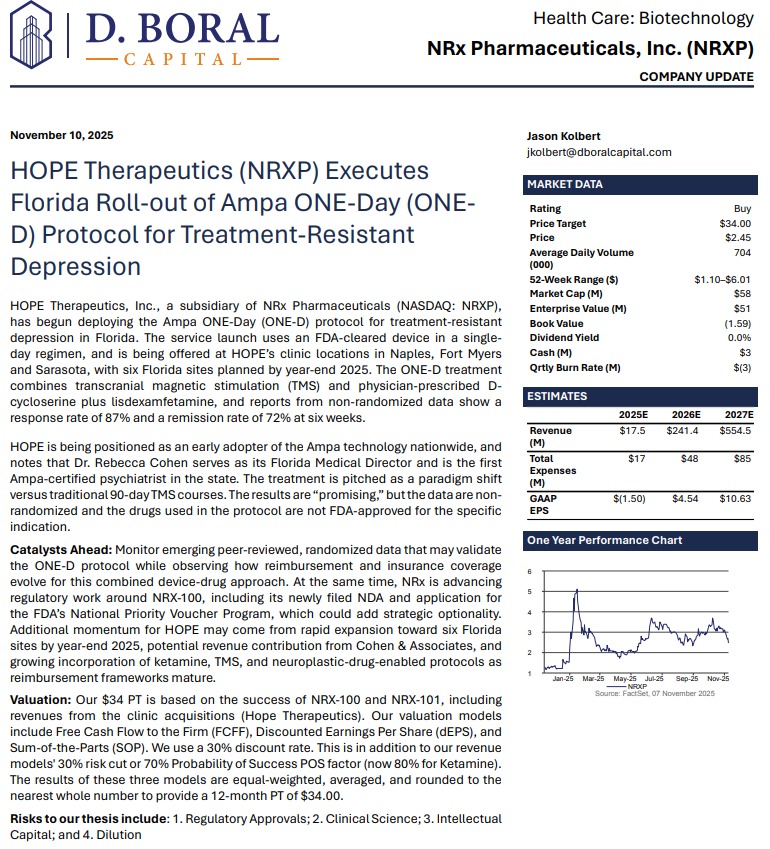
At the center of NRXP's drug pipeline is NRX-101, an FDA-designated Breakthrough Therapy targeting suicidal, treatment-resistant bipolar depression and chronic pain. The drug represents a potentially lifesaving option for the more than 13 million Americans who seriously consider suicide each year, according to the CDC.
NRXP is also advancing KETAFREE™, a preservative-free IV ketamine formulation, through a refiled Abbreviated New Drug Application (ANDA) with the FDA. This comes as the ketamine market—currently valued at $750 million—is projected to reach $3.35 billion globally by 2034.
Importantly, the FDA has granted approval of NRXP's Suitability Petition for its proposed strength of preservative-free ketamine, clearing the path for potential market entry amid ongoing national shortages of sterile ketamine formulations.
Strategic Expansion Through Dura Medical Acquisition
NRXP recently completed its acquisition of Dura Medical, a revenue-generating and EBITDA-positive network of precision psychiatry clinics along Florida's West Coast. The addition of Dura, alongside pending acquisitions such as Neurospa TMS and Cohen & Associates, positions NRXP to deliver an integrated continuum of mental health care spanning TMS, ketamine therapy, and advanced pharmacologic treatments for PTSD, depression, and chronic pain.
Robust Financial and Partnership Outlook
The company's growth strategy is supported by a $7.8 million debt financing deal with Universal Capital, LLC, aimed at expanding NRXP's HOPE Clinic network. In parallel, NRXP has accepted non-binding potential licensing terms for NRX-100, with the agreement representing over $300 million in milestone payments and tiered double-digit royalties—a major value inflection opportunity for investors.
More on The PennZone
Moreover, NRXP's partnership with Alvogen Pharmaceuticals supports the ongoing development and commercial readiness of NRX-101, further validating the company's clinical and commercial trajectory.
Analyst Confidence: H.C. Wainwright Issues "Buy" Rating and $40 Price Target
A recent analyst report from H.C. Wainwright & Co. titled "A Paradigm Shift in the Treatment of Depression with Suicidality" initiated coverage on NRx Pharmaceuticals with a Buy rating and a $34 price target. The report underscores NRXP's leadership in transforming how depression and suicidality are treated, leveraging FDA Fast Track and Breakthrough Therapy designations to accelerate commercialization.
"We view NRXP's approach as a paradigm shift in the treatment of depression with suicidality," wrote H.C. Wainwright analysts. "With novel drug candidates, a rapidly expanding clinic network, and strategic licensing opportunities, NRXP is uniquely positioned to capture substantial market share."
About NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
NRx Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company developing breakthrough therapeutics based on its NMDA receptor modulation platform for the treatment of central nervous system disorders, including suicidal bipolar depression, chronic pain, and PTSD.
Through its partnerships, innovative clinical pipeline, and integrated treatment model, NRXP is setting new standards in precision psychiatry and interventional mental health care.
For More Information:
Company: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com
Website: www.nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
A Major Step Forward: ONE-D Depression Treatment Launches in Florida
NRx Pharmaceuticals has initiated the first-in-Florida rollout of the ONE-D (One Day) Depression Treatment, in partnership with Ampa Health. The innovative protocol has shown breakthrough potential in achieving remission from treatment-resistant depression within a single day—an unprecedented leap forward for millions suffering from chronic depression.
Unlike traditional transcranial magnetic stimulation (TMS) therapies requiring up to 90 sessions over three months, the ONE-D approach combines a single-day TMS treatment with physician-prescribed compounds, including D-cycloserine, an active component of NRx's investigational NRX-101, and lisdexamfetamine. Early clinical data show an 87% response rate and 72% remission rate in nonrandomized studies.
The Ampa device is now available across NRXP HOPE Clinics in Sarasota, Naples, and Fort Myers, Florida—with six total locations expected by the end of 2025. This initiative marks one of the first national deployments of the Ampa ONE-D protocol.
More on The PennZone
- HBZBZL Unveils "Intelligent Ecosystem" Strategy: Integrating AI Analytics with Web3 Incubation
- Kaltra Launches Next-Gen MCHEdesign With Full Integration Into MCHEselect — Instant Simulation & Seamless Microchannel Coil Workflow
- A Well-Fed World, Youth Climate Save and PAN International Launch PHRESH: A Global Directory of Plant-Based Hunger Relief Organizations
- Guests Can Save 25 Percent Off Last Minute Bookings at KeysCaribbean's Village at Hawks Cay Villas
- Trump's Executive Order Rescheduling Cannabis: Accelerating M&A in a Multibillion-Dollar Industry
Breakthrough Therapeutic Portfolio: NRX-101 and KETAFREE™
At the center of NRXP's drug pipeline is NRX-101, an FDA-designated Breakthrough Therapy targeting suicidal, treatment-resistant bipolar depression and chronic pain. The drug represents a potentially lifesaving option for the more than 13 million Americans who seriously consider suicide each year, according to the CDC.
NRXP is also advancing KETAFREE™, a preservative-free IV ketamine formulation, through a refiled Abbreviated New Drug Application (ANDA) with the FDA. This comes as the ketamine market—currently valued at $750 million—is projected to reach $3.35 billion globally by 2034.
Importantly, the FDA has granted approval of NRXP's Suitability Petition for its proposed strength of preservative-free ketamine, clearing the path for potential market entry amid ongoing national shortages of sterile ketamine formulations.
Strategic Expansion Through Dura Medical Acquisition
NRXP recently completed its acquisition of Dura Medical, a revenue-generating and EBITDA-positive network of precision psychiatry clinics along Florida's West Coast. The addition of Dura, alongside pending acquisitions such as Neurospa TMS and Cohen & Associates, positions NRXP to deliver an integrated continuum of mental health care spanning TMS, ketamine therapy, and advanced pharmacologic treatments for PTSD, depression, and chronic pain.
Robust Financial and Partnership Outlook
The company's growth strategy is supported by a $7.8 million debt financing deal with Universal Capital, LLC, aimed at expanding NRXP's HOPE Clinic network. In parallel, NRXP has accepted non-binding potential licensing terms for NRX-100, with the agreement representing over $300 million in milestone payments and tiered double-digit royalties—a major value inflection opportunity for investors.
More on The PennZone
- Genuine Hospitality, LLC Selected to Operate Hilton Garden Inn Birmingham SE / Liberty Park
- Together We Dance Foundation Announces Transformational Support from NAC Have a Heart Foundation
- Harry Hayman Celebrates Years of WHYY Coverage, Partnership & Shared Commitment to Philadelphia
- Documentary "Prescription for Violence: Psychiatry's Deadly Side Effects" Premieres, Exposes Link Between Psychiatric Drugs and Acts of Mass Violence
- Stockwell Elastomerics expands micro molding capabilities
Moreover, NRXP's partnership with Alvogen Pharmaceuticals supports the ongoing development and commercial readiness of NRX-101, further validating the company's clinical and commercial trajectory.
Analyst Confidence: H.C. Wainwright Issues "Buy" Rating and $40 Price Target
A recent analyst report from H.C. Wainwright & Co. titled "A Paradigm Shift in the Treatment of Depression with Suicidality" initiated coverage on NRx Pharmaceuticals with a Buy rating and a $34 price target. The report underscores NRXP's leadership in transforming how depression and suicidality are treated, leveraging FDA Fast Track and Breakthrough Therapy designations to accelerate commercialization.
"We view NRXP's approach as a paradigm shift in the treatment of depression with suicidality," wrote H.C. Wainwright analysts. "With novel drug candidates, a rapidly expanding clinic network, and strategic licensing opportunities, NRXP is uniquely positioned to capture substantial market share."
About NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
NRx Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company developing breakthrough therapeutics based on its NMDA receptor modulation platform for the treatment of central nervous system disorders, including suicidal bipolar depression, chronic pain, and PTSD.
Through its partnerships, innovative clinical pipeline, and integrated treatment model, NRXP is setting new standards in precision psychiatry and interventional mental health care.
For More Information:
Company: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com
Website: www.nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
Filed Under: Health
0 Comments
Latest on The PennZone
- Teresa James & The Rhythm Tramps announce their new album and debut on MoMojo Records, 'Bad at Being Good'
- "Micro-Studio": Why San Diegans are Swapping Crowded Gyms for Private, One-on-One Training at Sweat Society
- Beycome Closes $2.5M Seed Round Led by InsurTech Fund
- VIP Vacations Invited to Travel Weekly's Annual Readers Choice Awards
- Tru by Hilton Columbia South Opens to Guests
- Christy Sports donates $56K in new gear to SOS Outreach to help kids hit the slopes
- "BigPirate" Sets Sail: A New Narrative-Driven Social Casino Adventure
- Phinge CEO Ranked #1 Globally by Crunchbase for the Last Week, Will Be in Las Vegas Jan. 4-9, the Week of CES to Discuss Netverse & IPO Coming in 2026
- Women's Everyday Safety Is Changing - The Blue Luna Shows How
- Microgaming Unveils Red Papaya: A New Studio Delivering Cutting-Edge, Feature-Rich Slots
- Harry Hayman IV & Gemini Consultants Announce Holiday Toys‑for‑Tots Giveaway with Retired Sixers
- 5-Star Duncan Injury Group Expands Personal Injury Representation to Arizona
- The End of "Influencer" Gambling: Bonusetu Analyzes Finland's Strict New Casino Marketing Laws
- AI-Driven Cybersecurity Leader Gains Industry Recognition, Secures $6M Institutional Investment, Builds Momentum Toward $16M Annual Run-Rate Revenue
- TRIO Heating, Air & Plumbing Now Ranks #1 in San Jose
- Milwaukee Job Corps Center Hosts Alumni Day, Calls Alumni to Action on Open Enrollment Campaign
- Ezra Wohlgelernter Installed Philadelphia Bar Association Chancellor
- Power Couple Launches "Happy Habits Events" After Best of Philly Win, Pandemic Loss, and Setbacks
- Golden Paper Identifies Global Growth in Packaging Papers and Upgrades Its High-End Production Capacity
- Champagne, Caviar Bumps & Pole Performances — Welcome the New Year Early with HandPicked Social Club